Method for continuously preparing emamectin benzoate and intermediate thereof
A technology of emamectin and benzoate, applied in the field of continuous preparation of emamectin benzoate and its intermediates, capable of solving many side reactions, high frequency of solvent replacement, and long reaction time And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
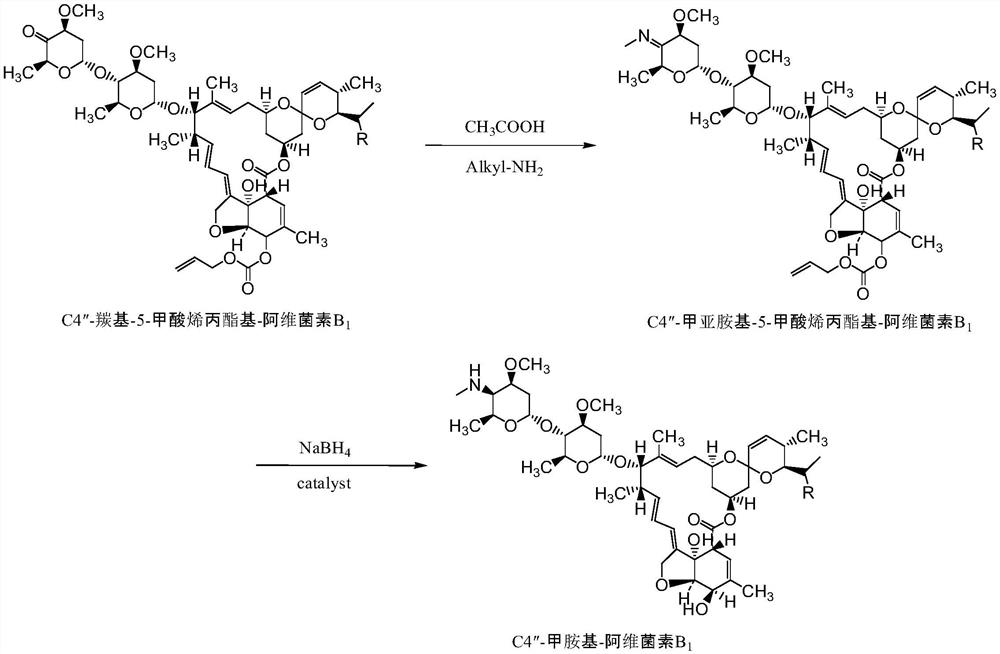
[0039] A preparation of C4 "-methylamino-5-formyl allyl-abamectin B 1 method, the specific steps are as follows:
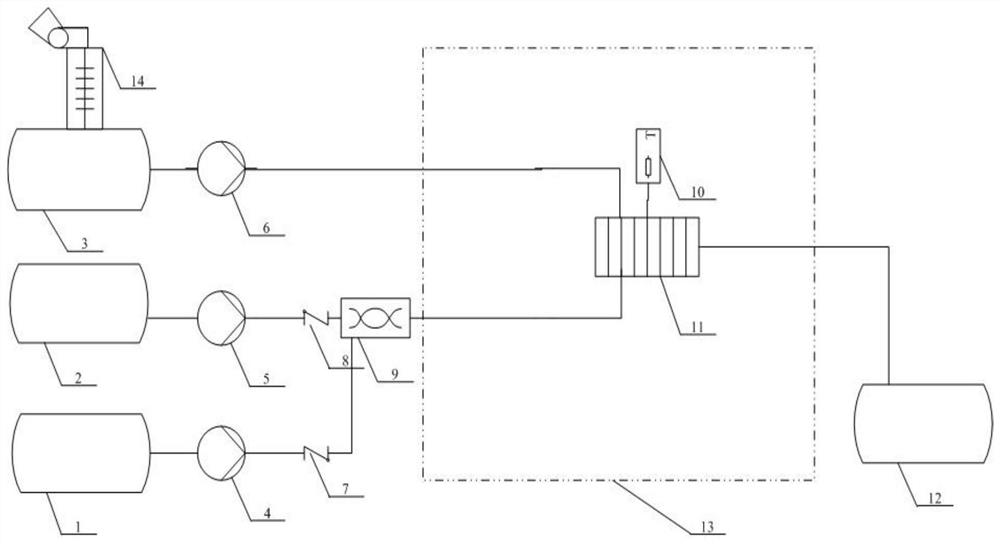
[0040] C4"-formimino-5-allyl-carboxylate-abamectin B 1 Dissolved in dichloromethane to form a homogeneous solution, pumped into the microchannel modular mixing device with methanol, and then pumped the mixed solution with the reducing agent into the microchannel modular reaction device, C4”-formimino-5-formic acid Allyl ester-abamectin B 1: Dichloromethane: Methanol: The molar ratio of the reducing agent is 1:40:10:5. In the microreactor of the microchannel modular reaction device, the reaction temperature is controlled to be 0°C and the reaction residence time is 5s. Compound C4"-methylamino-5-allyl carboxylate-abamectin B 1 .
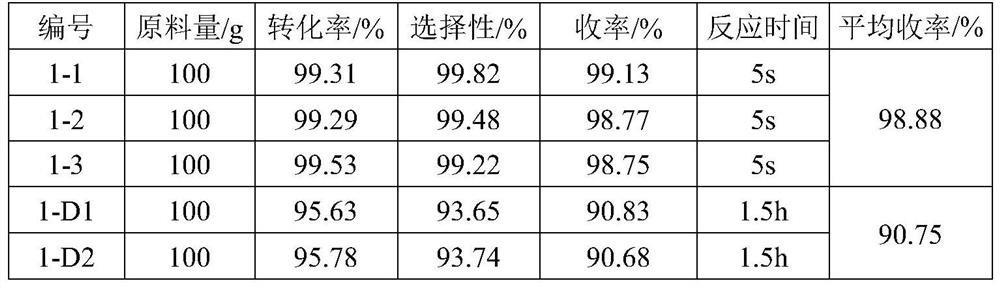
[0041] Parallel experiments were carried out in 3 groups, and the original method was carried out in 2 groups of control experiments.
[0042] Controlled experiment (traditional method) parallel 2 groups:
[0043] 1) Add C4"-formimi...
Embodiment 2
[0049] A preparation of C4 "-methylamino-5-formyl allyl-abamectin B 1 method, the specific steps are as follows:
[0050] C4"-formimino-5-allyl-carboxylate-abamectin B 1 Dissolved in dichloromethane to form a homogeneous solution, pumped into the microchannel modular mixing device with methanol, and then pumped the mixed solution with the reducing agent into the microchannel modular reaction device, C4”-formimino-5-formic acid Allyl ester-abamectin B 1 : Dichloromethane: Methanol: The molar ratio of reducing agent is 1:40:30:7, in the microreactor of the microchannel modular reaction device, the reaction temperature is controlled to be 0°C, the reaction residence time is 5s, and the sample is detected to generate Compound C4"-methylamino-5-allyl carboxylate-abamectin B 1 .
[0051] Parallel experiments were carried out in 3 groups, and the original method was carried out in 2 groups of control experiments.
[0052] Controlled experiment (traditional method) parallel 2 gro...
Embodiment 3
[0059] A method for preparing C4 "-methylamino-5-formic acid allyl group-abamectin B1, the specific steps are as follows:
[0060] C4"-formimino-5-allyl-carboxylate-abamectin B 1 Dissolved in dichloromethane to form a homogeneous solution, pumped into the microchannel modular mixing device with methanol, and then pumped the mixed solution with the reducing agent into the microchannel modular reaction device, C4”-formimino-5-formic acid Allyl ester-abamectin B 1 : Dichloromethane: Methanol: The molar ratio of the reducing agent is 1:40:30:7, the reaction temperature is controlled to be 15°C and the reaction residence time is 10s in the microreactor of the microchannel modular reaction device, and the sample is detected to generate Compound C4"-methylamino-5-allyl carboxylate-abamectin B 1 .
[0061] Parallel experiments were carried out in 3 groups, and the original method was carried out in 2 groups of control experiments.
[0062] Controlled experiment (traditional method...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com