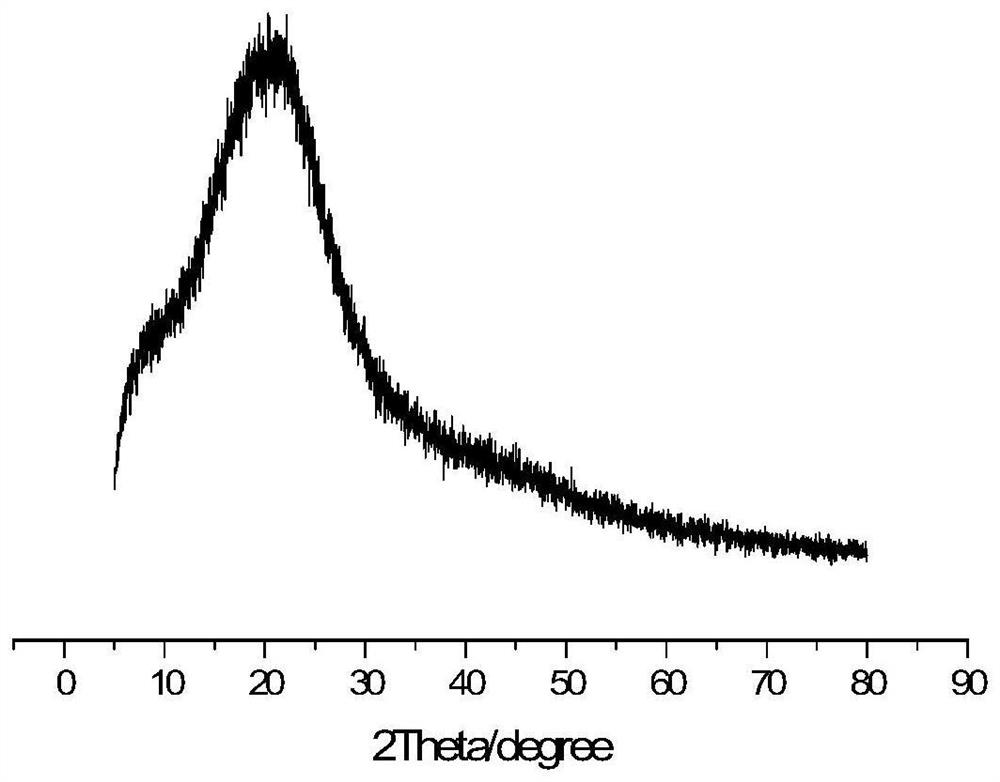
Carbazole porous polymer containing 8-hydroxyquinoline group as well as preparation method and application of carbazole porous polymer
A porous polymer, hydroxyquinoline technology, used in instruments, analytical materials, measuring devices, etc., can solve the problems of expensive precious metals, increased detection costs, poor stability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
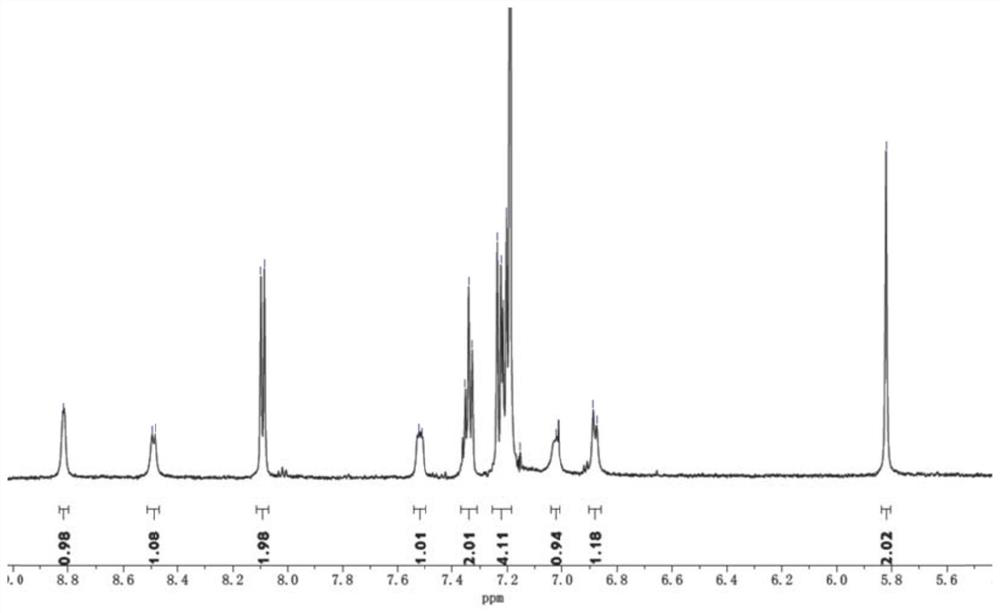
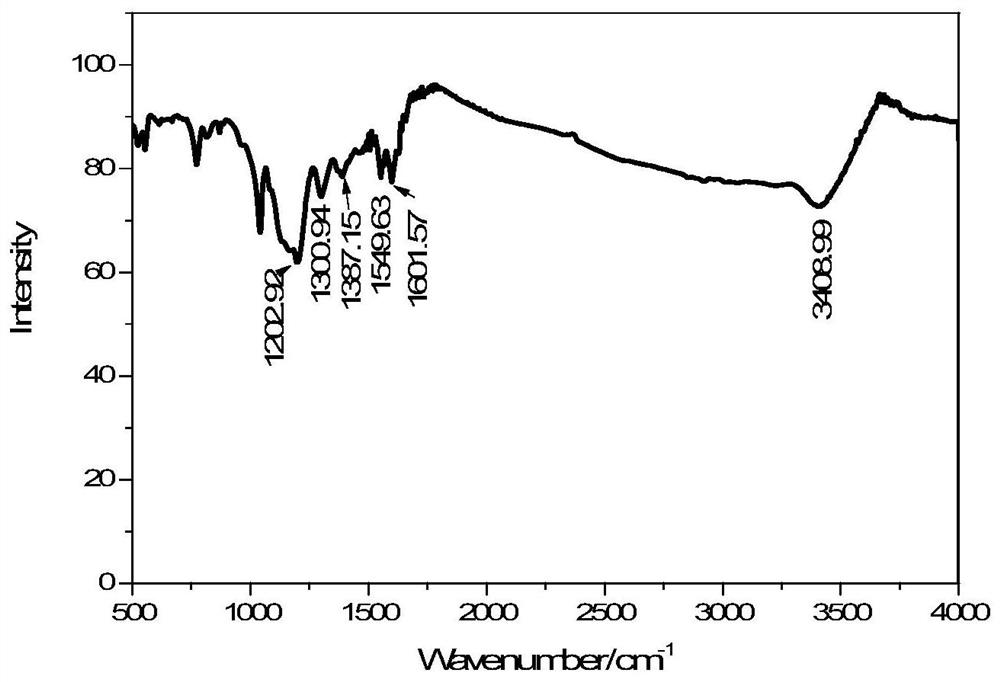
[0040](1) Synthesis of 5-chloromethyl-8-hydroxyquinoline hydrochloride: take 8-hydroxyquinoline (10mmol, 1.45g) and join in the there-necked flask containing 20ml paraformaldehyde, magnetic stirring and heating to 80°C, 10ml of concentrated hydrochloric acid (38% HCl, 120mmol) was slowly added dropwise to the reaction liquid, kept at 80°C for 6h after the dropwise addition, cooled naturally to room temperature, filtered with suction to obtain a filter cake, washed three times with 50ml of acetone, and vacuumed at 45°C Dry to obtain a yellow powder with a yield of 63%.
[0041] (2) Synthesis of 5-(N-methylenecarbazole)-8-hydroxyquinoline: Weigh carbazole (5mmol, 0.835g) and NaH (10mmol, 0.24g) and dissolve it in 20mlDMSO, stir at room temperature until No bubbles were generated. After the mixture was filtered, the reaction solution was filtered and placed in a three-necked flask. KOH (10 mmol, 0.56 g) was added to the filtrate, and magnetically stirred for 30 minutes. Weigh 5-...
Embodiment 2
[0053] Embodiment 2 and embodiment 1 are prepared identically, and difference is: the mol ratio of 8-hydroxyquinoline and hydrochloric acid (HCl, mass fraction 36%) is 1:10 in the step (1), and reaction temperature is 65 ℃, time is 5h; the mol ratio of 5-chloromethyl-8-hydroxyquinoline and carbazole in step (2) is 1:1.5, the mol ratio of carbazole and NaH, KOH is 1:1:1, and the reaction temperature is 80 ℃, the heating reaction time is 5h; in step (3), the molar ratio of 5-(N-methylenecarbazole)-8-hydroxyquinoline and trichlorotriazine to cyanuric chloride is 2:2, and the reaction temperature is 100 ℃, the reaction time is 72h, the molar ratio of 5-(N-methylenecarbazole)-8-hydroxyquinoline and methanesulfonic acid is 1:5.
Embodiment 3
[0055] Embodiment 3 and embodiment 1 are prepared identically, and difference is: the mol ratio of 8-hydroxyquinoline and hydrochloric acid (HCl, mass fraction 38%) is 1:20 in step (1), and reaction temperature is 80 ℃, time is 5h; the mol ratio of 5-chloromethyl-8-hydroxyquinoline and carbazole in step (2) is 1:1.2, the mol ratio of carbazole and NaH, KOH is 1:3:3, and the reaction temperature is 80 ℃, the heating reaction time is 2h; the molar ratio of 5-(N-methylenecarbazole)-8-hydroxyquinoline and trichlorotriazine cyanuric chloride in step (3) is 3:2, and the reaction temperature is 150 ℃, the reaction time is 12h, the molar ratio of 5-(N-methylenecarbazole)-8-hydroxyquinoline and methanesulfonic acid is 1:80.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com