Cefradine dispersible tablet and preparation method thereof
A technology for cefradine and dispersible tablets, applied in the field of cefradine dispersible tablets and their preparation, can solve the problems of easy generation of degraded impurities, slow growth and reduction of degraded impurities and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
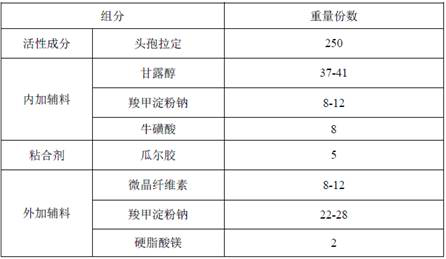
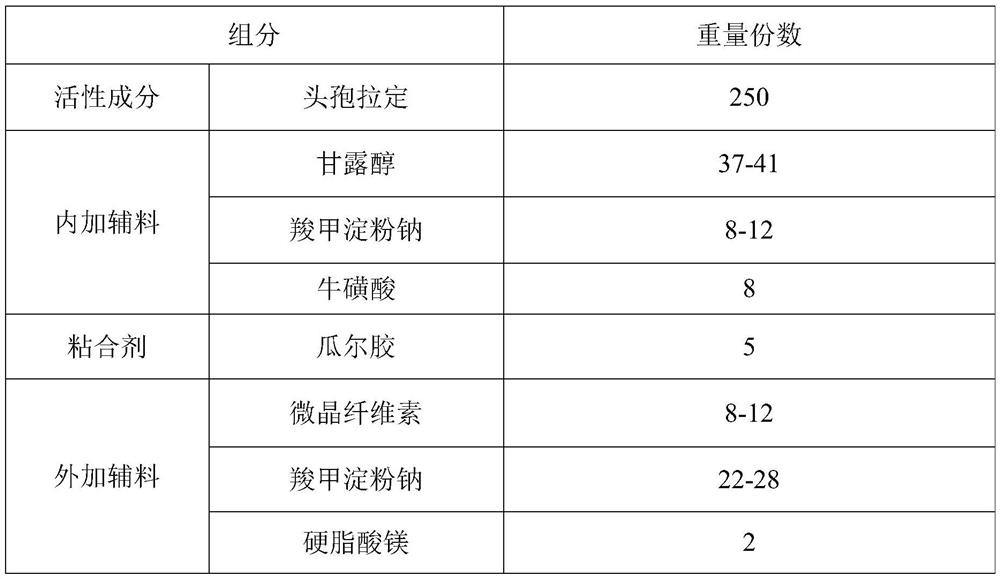
[0054] Embodiment 1: Cephradine dispersible tablet
[0055] 1. Prescription composition:
[0056]
[0057] Feed intake by the prescription quantity of 1000, prepare cephradine dispersible tablet, the same below
[0058] 2. Preparation method:
[0059] (1) Preparation of binder solution: the binder guar gum is dissolved in water to prepare a guar gum solution with a concentration of 5.0% (w / w);
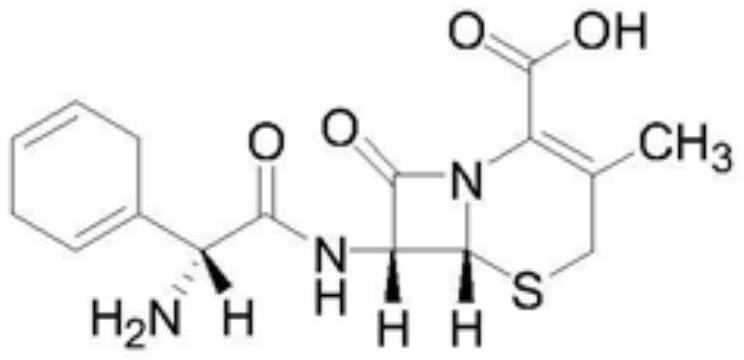
[0060] (2) Premixing and wet granulation: first add cephradine, mannitol, sodium starch glycolate, and taurine into a wet mixing granulator for premixing, then add the binder solution under stirring conditions, and wet granulate grain;
[0061] (3) Drying: Use a fluidized granulator to dry the wet granules, adopt low-temperature drying technology, set the inlet air temperature to 45°C, the outlet air temperature to 38°C, and the material temperature to 41°C. Take samples to measure the moisture, and the moisture reaches 1.2 %, after drying, the drying time is about 35 minutes; ...
Embodiment 2
[0069] Embodiment 2: Cephradine dispersible tablet
[0070] 1. Prescription composition:
[0071]
[0072] 2, preparation method: with embodiment 1.
[0073] 3. Test results:
[0074] The prepared cephradine dispersible tablets were tested for friability, uniformity of dispersion and dissolution, and the results are shown in table 3.
[0075] Table 3: Embodiment 2 Cephradine Dispersible Tablets carry out friability, uniformity of dispersion, and dissolution results
[0076]
Embodiment 3
[0077] Embodiment 3: Cephradine dispersible tablet
[0078] 1. Prescription composition:
[0079]
[0080] 2, preparation method: with embodiment 1.
[0081] 3. Test results:
[0082] The prepared cephradine dispersible tablets were tested for friability, uniformity of dispersion and dissolution, and the results are shown in table 43.
[0083] Table 4: Embodiment 3 Cephradine Dispersible Tablets carry out friability, uniformity of dispersion, and dissolution results
[0084]
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com