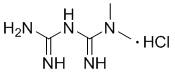
Method for controlling genotoxic impurities in metformin hydrochloride sustained release tablet preparation process
A metformin hydrochloride and genotoxic technology, which is applied in the direction of making medicines into special physical or taking forms of devices, pill delivery, pharmaceutical formulations, etc. The effect of strong operability and simple control method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1 Investigation of Key Prescription Factors in Preparation Technology
[0022] Preliminary studies have found that no genotoxic impurities were detected in metformin hydrochloride API and various excipients N -Nitrosodimethylamine, and does not generate impurities under acid, alkali, oxidative degradation conditions N -Nitrosodimethylamine, so we conduct a compatibility study of raw materials and excipients to investigate the influence of prescription factors in the preparation process. N - The content determination of nitrosodimethylamine adopts the analytical method of GC-MS. The results are shown in Table 1.
[0023] Table 1 Compatibility study results of raw materials and excipients
[0024]
[0025] The research results showed that: ① the same batch of raw materials, the higher the nitrite content of hypromellose in the mixed powder of excipients, the higher the content of hypromellose in the sample N - The higher the content of nitrosodimethylamine;...
Embodiment 2
[0027] Example 2 The formulation of the limit of impurity dimethylamine content in metformin hydrochloride crude drug
[0028] 1. Sample Preparation
[0029] According to the proportion of the prescription, the metformin hydrochloride bulk drug with different dimethylamine content and the sustained-release matrix material hypromellose K100M, microcrystalline cellulose and sodium carboxymethylcellulose were placed in a high-efficiency wet granulator and mixed for 5- Mix for 10 minutes, then add 20% ethanol solution to prepare wet granules; wet granules are boiled and dried in a fluidized bed below 40°C (moisture ≤ 2.0%), dry granules are granulated with a 20-mesh oscillating granulator, and finally granulated The granules after granulation are uniformly mixed with magnesium stearate and hypromellose K100M in a mixer, and compressed into tablets. Simultaneously carry out research on influencing factors to investigate the impact of dimethylamine content in raw materials on produ...
Embodiment 3
[0034] Embodiment 3 Limit formulation of impurity nitrite content in the auxiliary material hypromellose
[0035] 1. Sample Preparation
[0036] According to the proportion of the prescription, the metformin hydrochloride bulk drug and the slow-release matrix material hypromellose K100M, microcrystalline cellulose, and sodium carboxymethylcellulose with different nitrite contents were placed in a high-efficiency wet granulator and mixed for 5- Mix for 10 minutes, then add 20% ethanol solution to prepare wet granules; wet granules are boiled and dried in a fluidized bed below 40°C (moisture ≤ 2.0%), dry granules are granulated with a 20-mesh oscillating granulator, and finally granulated The granules after granulation are uniformly mixed with magnesium stearate and hypromellose K100M in a mixer, and compressed into tablets. At the same time, the influence factor test was carried out to investigate the effect of the nitrite content in the auxiliary material hypromellose on the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com