Easily degradable responsive core-crosslinkable amphiphilic block polymer, preparation method thereof and application of amphiphilic block polymer as drug carrier
An amphiphilic block and easily degradable technology, which is used in medical preparations, pharmaceutical formulations, and emulsion delivery of non-active ingredients, can solve problems such as long-term toxicity, and achieve high drug loading, mild reaction conditions, and adverse reactions. less reactive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
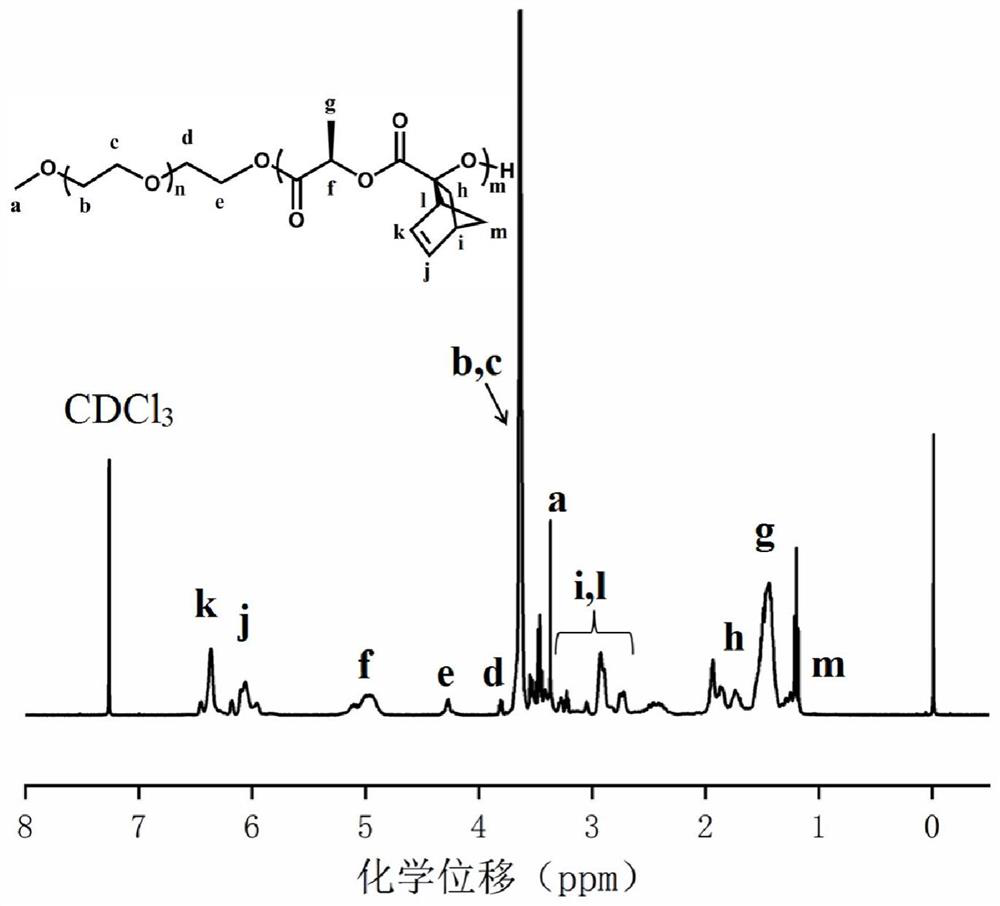
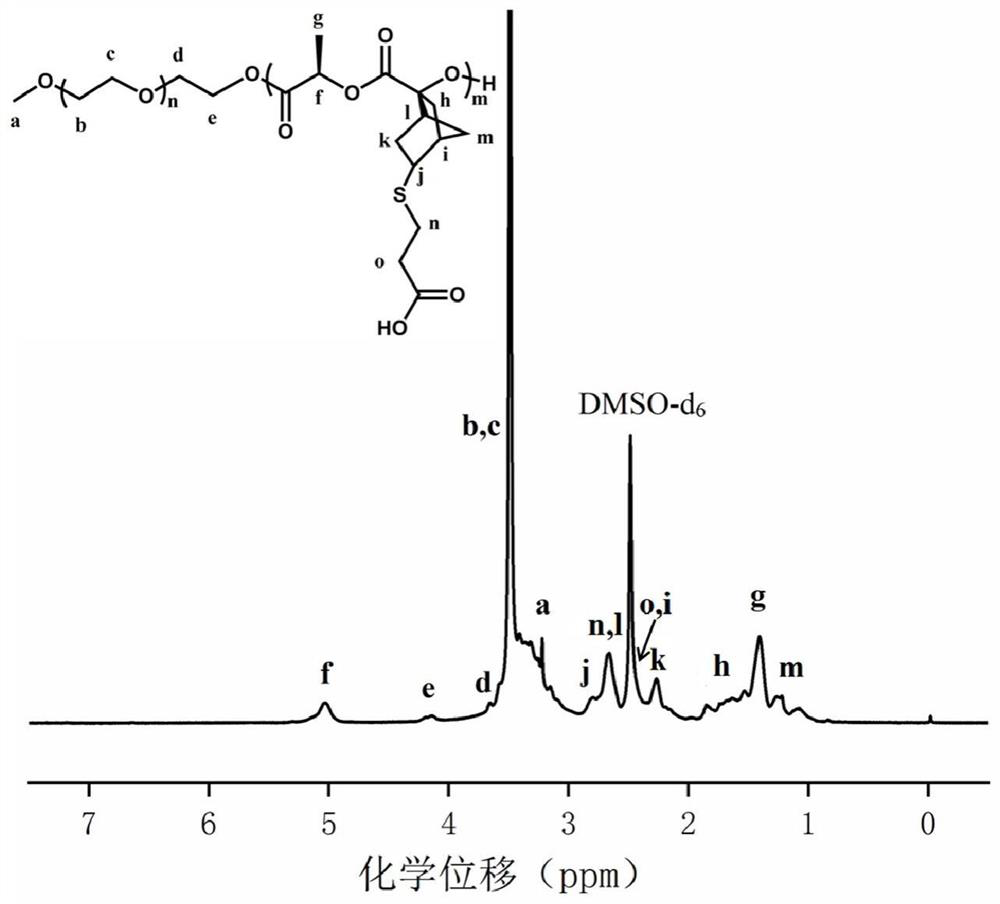
[0065] Synthesis of polyethylene glycol monomethyl ether-b-polylactide block polymer mPEG-b-P(LA-SS-Py) containing dithiopyridine side groups and carboxyl side groups:
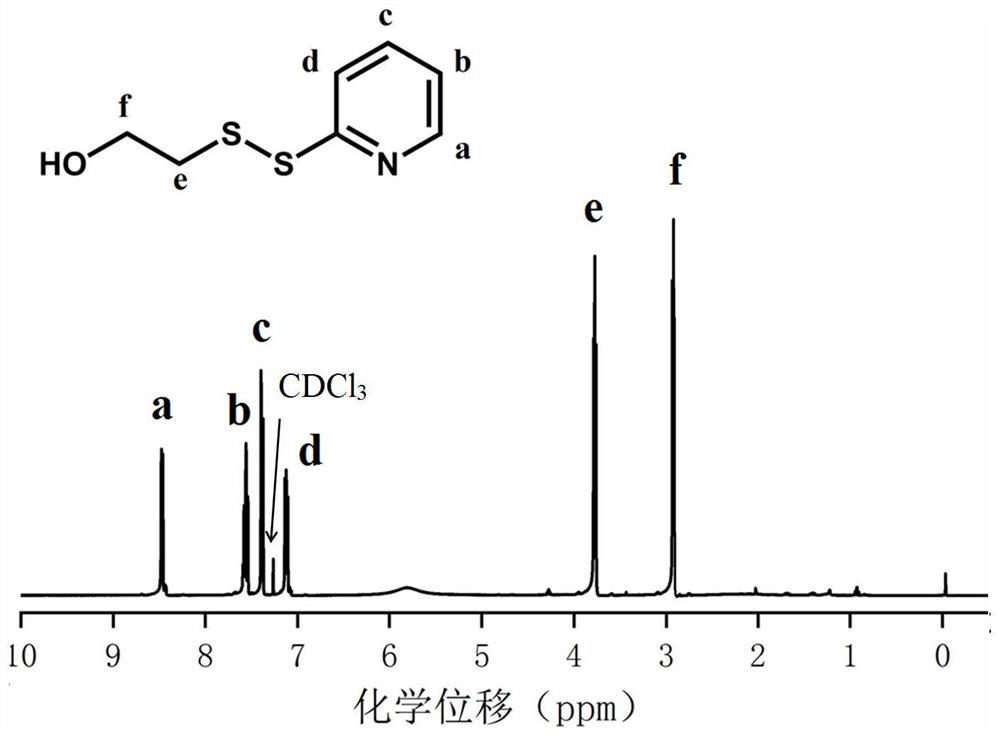
[0066] 1. Synthesis of ethyl hydroxydithiopyridine compound (HO-SS-Py):
[0067] Weigh DTDP (5g, 0.022mol) and 0.3mL of glacial acetic acid in a 50mL round-bottomed flask with a magnet, dissolve it completely with 25mL of methanol, and add dropwise to the mixture containing 2-mercaptoethanol (0.886 g, 11.36 mmol) in 5 mL of methanol solution, after the addition was completed, the reaction mixture was stirred at room temperature for 3 to 4 hours, then the stirring was stopped, and the solvent was evaporated to obtain the crude product of yellow oil, which was purified by silica gel column chromatography, Using silica gel as the stationary phase and a mixture of ethyl acetate / petroleum ether as the eluent, excess DTDP first appeared in a 15% ethyl acetate / petroleum ether mixture, then the polarity of the eluent ...
Embodiment 2
[0075] Preparation of micelles:
[0076] 1. Preparation and size adjustment of blank non-crosslinked micelles:
[0077] Take 10 mg of the amphiphilic block polymer obtained in Example 1 above, dissolve it in 4 mL of DMF, then add it dropwise to 6 mL of deionized water, stir for 4 h, transfer it to a dialysis bag (MWCO=3500 Da), and use Dialyze with deionized water, change the water every 3 hours, filter through a 0.45 μm filter membrane after 12 hours, freeze-dry, and finally obtain amphiphilic block copolymer blank non-crosslinked micelles. Figure 7 Middle a is the DLS diagram of the blank non-crosslinked micelles, the average particle size is 84nm, and the PDI is 0.126.
[0078] 2. Preparation of blank cross-linked micelles:
[0079] Take 10 mg of the amphiphilic block polymer obtained in Example 1 above, dissolve it in 4 mL of DMF, and then add it dropwise to a solution containing (30% relative to the dithiopyridine group) dithiothreose under a nitrogen atmosphere. Alco...
Embodiment 3
[0085] Micellar Stability Test:
[0086] Take the four kinds of micelles prepared in Example 2 and configure them into two parts of 1 mg / mL solution. At room temperature, dilute them by 3 times and 10 times with DMF solvent respectively. Distribution, after being diluted with the organic solvent DMF, the particle size changes, such as Figure 8 As shown, the particle size of the cross-linked micelles only increased slightly after being diluted 3 times and 10 times with DMF solvent, which indicated that it only swelled to a certain extent under the dilution of organic solvents, and there was no dissociation phenomenon, maintaining a relatively good stability. On the contrary, after the uncrosslinked micelles were diluted 3 times by DMF, larger particles appeared instead, which indicated that under the dilution of organic solvents, the uncrosslinked micelles dissociated and re-aggregated, while the diluted Uncrosslinked micelles were completely dissociated after ten times and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com