Separation and purification method of recombinant protein
A recombinant protein, separation and purification technology, applied in the field of protein chemistry, can solve the problems of long process route, high production cost, high aggregate content, etc., and achieve the effects of improving purification efficiency, simplifying operation, and reducing protein adsorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
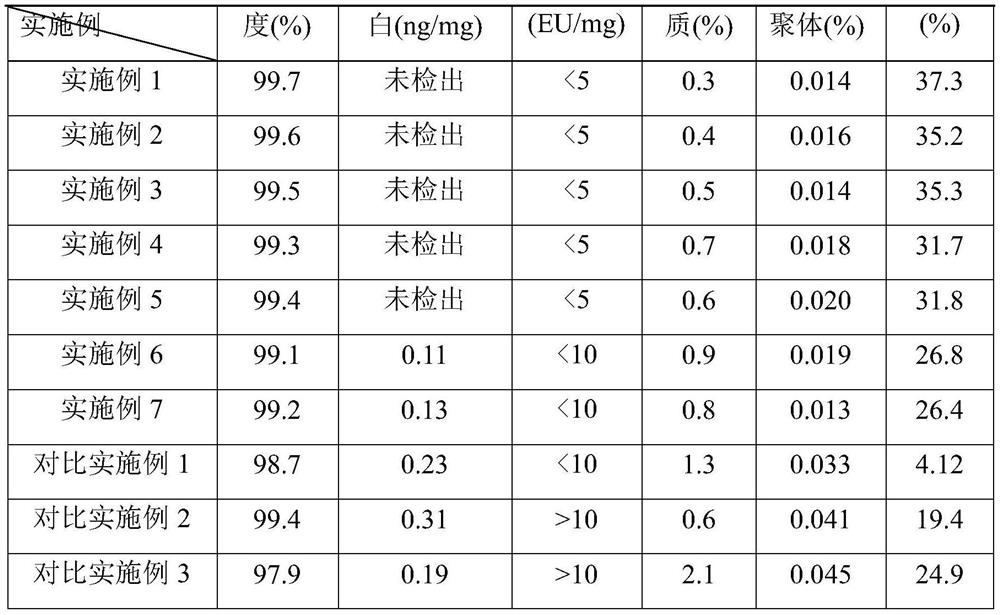
Embodiment 1
[0071] A. Ultrafiltration: The primary purified sample (120.5L, 0.25g / L) containing insulin glargine precursor was clarified and filtered through cellulose membrane regenerated cellulose P2C-100-C01 100KD tangential flow membrane filtration, concentrated 30 times Finally, replace the concentrated protein solution and 0.1M ammonium sulfate buffer solution at a volume ratio of 1:4 with an ultrafiltration membrane bag for at least 3 times, and combine the filtrate, which is the filtered sample of insulin glargine precursor, which is collected and stored in 2-8 In ℃ environment, the yield is 95.2%;
[0072] B. Digestion: Use bovine trypsin to digest the filtered sample containing insulin glargine precursor obtained in step A, add 35.85 mg of bovine trypsin, adjust the pH to 9.0, control the temperature at 4°C, digest for 12 hours, and adjust the pH to 3 .0 to terminate the enzyme digestion, and obtain the enzyme digestion solution, which is stored in an environment of 2-8°C, and t...
Embodiment 2
[0078] A. Ultrafiltration: The primary purification sample (120.9L, 0.25g / L) containing insulin precursor was clarified and filtered by PLC-100-C01200KD tangential flow membrane filtration, concentrated 20 times, and concentrated by ultrafiltration membrane bag The protein solution and 0.1M ammonium sulfate buffer were replaced at least 4 times at a volume ratio of 1:3, and the combined filtrate was the filtered sample of insulin glargine precursor, which was collected and stored in an environment of 2-8°C, with a yield of 94.8%;
[0079] B. Digestion: Use bovine trypsin to digest the filtered sample obtained in step A, add 40.94 mg of bovine trypsin, adjust the pH to 9.5, control the temperature at 8°C, digest for 10 hours, adjust the pH to 3.0 to stop the digestion, and obtain Enzyme cutting solution, the enzyme cutting solution is stored in an environment of 2-8°C, and the yield is 60.1%;
[0080] C. Reverse-phase chromatography: Use Source 30RPC to pack a column with a col...
Embodiment 3
[0085] A. Ultrafiltration: The primary purification sample (120.4L, 0.25g / L) containing the insulin glargine precursor was clarified and filtered through polyethersulfone P2B-100-A05 300KD, tangential flow membrane filtration, concentrated 50 times, Replace the concentrated protein solution and 0.1M ammonium sulfate buffer at a volume ratio of 1:5 for at least 3 times with an ultrafiltration membrane bag, combine the filtrates, collect and store them in an environment at 2-8°C, with a yield of 94.8%;
[0086] B. Digestion: Use porcine trypsin to digest the filtered sample obtained in step B, add 31.70 mg of porcine trypsin, adjust the pH to 8.0, control the temperature at 6°C, digest for 13 hours, adjust the pH to 3.0 to stop the digestion, and obtain Enzyme cutting solution, the enzyme cutting solution is stored in an environment of 2-8°C, and the yield is 60.3%;
[0087]C. Reversed-phase chromatography: use Source 30RPC to pack a column with a column diameter of 10cm and a h...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com