Method for purifying recombinant protein
A technology of recombinant protein and protein, applied in the field of protein chemistry, can solve the problems of long process route, complicated operation, narrow application range, etc., and achieve the effect of improving purification efficiency, low protein adsorption amount, and wide application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
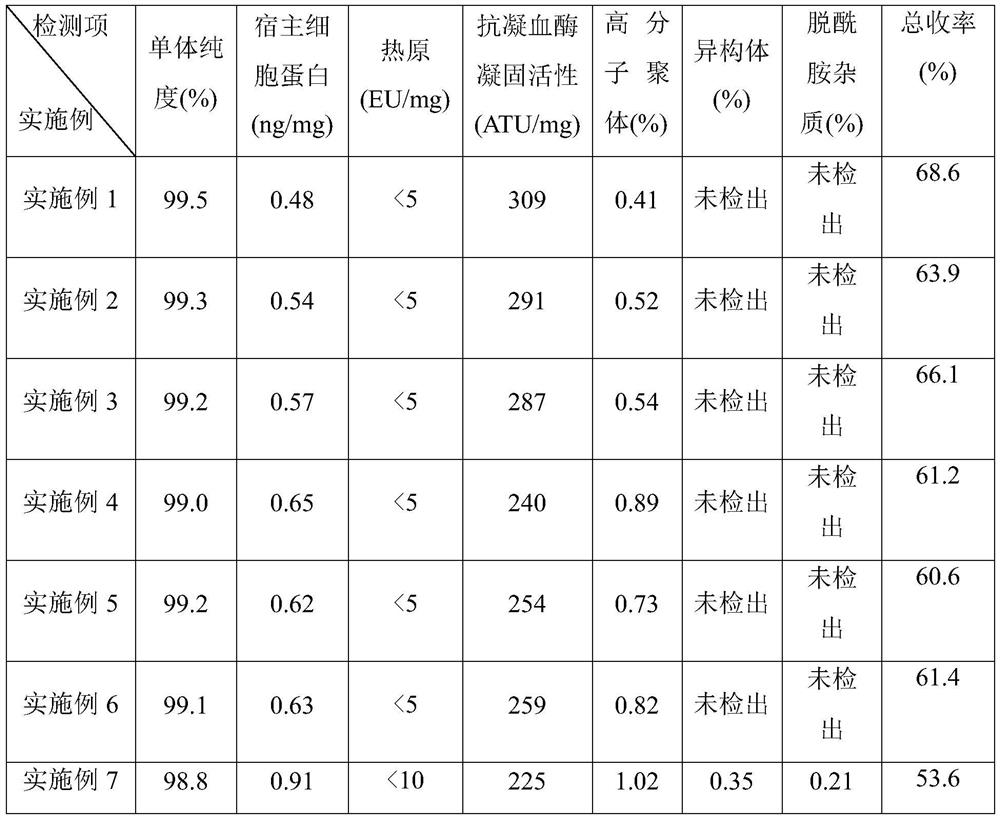
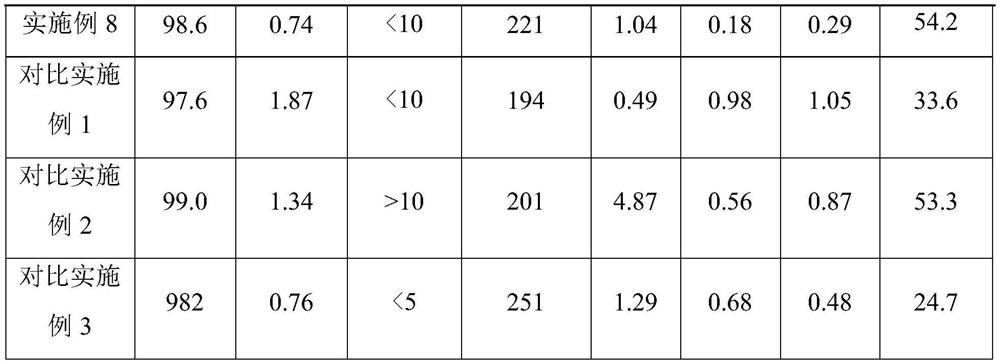
[0066] A. Ultrafiltration: The primary purification sample (103.4L, 0.3g / L) is clarified and filtered through a cellulose membrane PLCHK-C 100KD tangential flow membrane filter. The solution and the buffer were replaced at a volume ratio of 1:4, and the combined filtrate was the filtered sample of leukocyte inhibitory factor and hirudin chimeric protein, which was collected and stored at 2-8°C, with a yield of 93.6%;
[0067] B. Hydrophobic chromatography: At room temperature, use Phenyl Sepharose High Performance column with a diameter of 10cm and a height of 20cm (column bed volume 1.57L), and then use 5mM Tris-HCl and 300mM ammonium sulfate buffer solution with a pH of 7.5 After equilibration, load the filtered sample containing leukocyte inhibitory factor and hirudin chimeric protein obtained in step A. After loading the sample, wash the chromatography column with 5mM Tris-HCl and 300mM ammonium sulfate buffer solution with a pH of 7.5 until the effluent is detected. The v...
Embodiment 2
[0072] A. Ultrafiltration: The primary purification sample (107.7L, 0.3g / L) was clarified and filtered through a polyethersulfone membrane BioMax 100KD tangential flow membrane filter. After being concentrated 20 times, the concentrated protein solution was purified by ultrafiltration membrane bag Replace with buffer solution at a volume ratio of 1:5, and combine the filtrate, which is the filtered sample of leukocyte inhibitory factor and hirudin chimeric protein, collected and stored in an environment of 2-8°C, with a yield of 92.6%;
[0073] B. Hydrophobic chromatography: At room temperature, use Phenyl Sepharose Fast Flow to pack the column with a diameter of 10cm and a height of 20cm (column bed volume 1.57L), and then use 5mM Tris-HCl and 300mM ammonium sulfate buffer solution with a pH of 7.0 After equilibration, load the filtered sample containing leukocyte inhibitory factor and hirudin chimeric protein obtained in step A. After loading the sample, wash the chromatograp...
Embodiment 3
[0078] A. Ultrafiltration: The primary purification sample (99.5L, 0.3g / L) is clarified and filtered through a cellulose membrane PLCHK-C 200KD tangential flow membrane filter. The solution and the buffer were replaced at a volume ratio of 1:3, and the combined filtrate was the filtered sample of leukocyte inhibitory factor and hirudin chimeric protein, which was collected and stored at 2-8°C, with a yield of 93.4%;
[0079] B. Hydrophobic chromatography: At room temperature, use Phenyl Sepharose High Performance column with a diameter of 10cm and a height of 20cm (column bed volume 1.57L), and then use 5mM Tris-HCl and 300mM ammonium sulfate buffer with a pH of 8.0 After equilibration, load the filtered sample containing leukocyte inhibitory factor and hirudin chimeric protein obtained in step A. After loading the sample, wash the chromatography column with 5mM Tris-HCl and 300mM ammonium sulfate buffer solution with pH 8.0 until the effluent is detected. The value is the bas...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com