N-acyl homoserine lactone acyltransferase coding gene aigA and application thereof
A technology based on acyl homoserine and lactone acyl, which can be used in applications, genetic engineering, plant genetic improvement, etc. It can solve problems such as microbial resistance, pesticide and antibiotic environmental safety, and human and animal health threats, and achieve good quenching activity , good degradation effect, broad-spectrum quenching effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1 Acquisition and identification of AHLs degradation genes
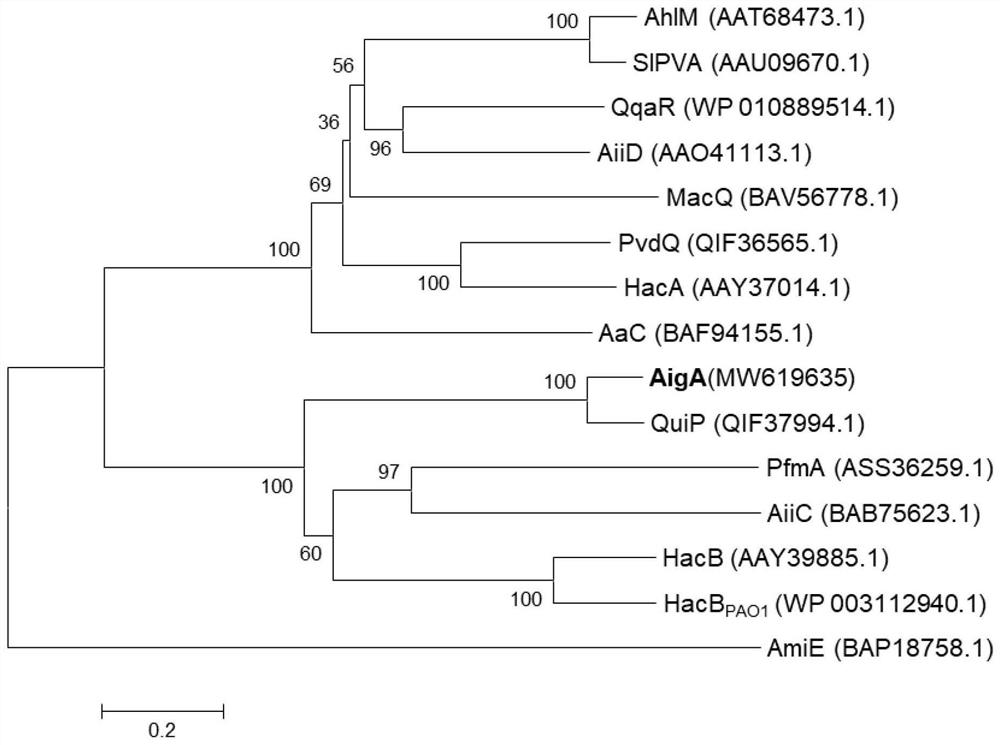
[0054] According to the whole genome sequencing results of Pseudomonas nitroreduction strain HS-18 in the previous work, the gene aigA, which may encode N-acyl homoserine lactone acyltransferase, was found by genome annotation and bioinformatics comparison. The software MEGA 5.10 was used to align the amino acid sequences of AigA and the currently known N-acylhomoserine lactone acyltransferases, and ClustalX1.8.3 and neighbor-joining method were used to conduct phylogenetic analysis and construct an evolutionary tree. The result is as figure 2 It was shown that AigA belongs to AHLs acylase of Penicillin G acylase family, and has the highest similarity (82.29%) with the amino acid sequence of QuiP in known AHLs acylase.
[0055] The primers were designed to amplify and construct the primers for aigA to be inserted into the broad host vector pBBR1 and the protein prokaryotic expression vector pET32a re...
Embodiment 2
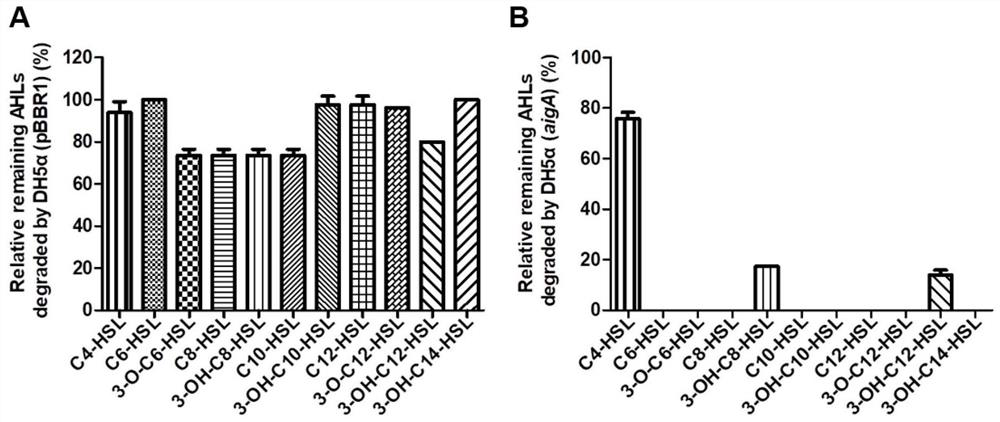
[0060] Example 2 Detection of AHLs quenching activity by recombinant bacteria DH5α(aigA)
[0061] AHLs degradation system setting: the DH5α(aigA) successfully constructed overnight was cultured in LB liquid medium to obtain OD 600 = 1.0 seed solution, add an equal volume of fresh LB liquid medium and exogenous AHLs with different carbon chain lengths and substituents with a final concentration of 10-50 μM and MOPS with a final concentration of 50 mM to prepare a degradation system (different AHLs signal According to the different intensity of the reporter strain color development, the appropriate concentration was selected), cultured at 37 ° C, 200 rpm incubator for 36 h, with DH5α (pBBR1) bacterial liquid and LB liquid medium without bacterial liquid as the control. Next, the culture solution was extracted with an equal volume of ethyl acetate, and the content of AHLs remaining in 10 μl of the ethyl acetate extract was detected by the reporter strain.
[0062] Quantitative d...
Embodiment 3
[0065] Example 3 Prokaryotic expression of AigA protein
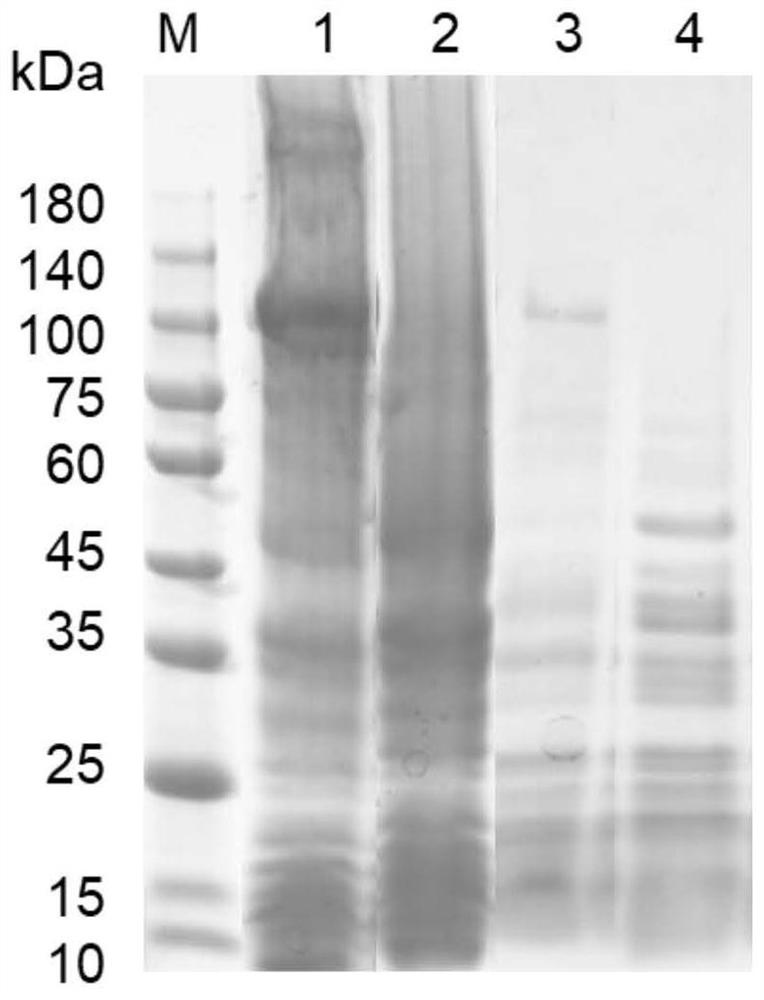
[0066] The successfully constructed recombinant bacteria BL21(DE3)(pET32a-aigA) was cultured overnight in LB liquid medium supplemented with a final concentration of 100 μg / ml ampicillin, and the seed solution was obtained by culturing at 37°C and 200rpm in a constant temperature shaker. Then, the seed solution was added to fresh LB liquid medium containing a final concentration of 100 μg / ml ampicillin at a ratio of 1:100, and cultivated to OD in a constant temperature shaker at 37 °C and 200 rpm. 600 = 0.6-0.8, add IPTG at a final concentration of 0.5 mM for induction, and culture overnight at 18°C in a constant temperature shaker at 200 rpm. The cells cultured overnight were collected and disrupted, and the expression of AigA was identified by SDS-PAGE electrophoresis. according to image 3The results showed that AigA carrying His tag could be expressed in normal prokaryotic cells, and its size was about 112.27kDa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com