Cationic complex liposome influenza vaccine as well as preparation method and application method thereof
A technology of cationic complexes and cationic liposomes, which is applied in the field of nanomaterial technology and the field of vaccines, can solve the problems of many side effects, difficult immune response, poor safety, etc., and achieve the effect of promoting antigen uptake
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
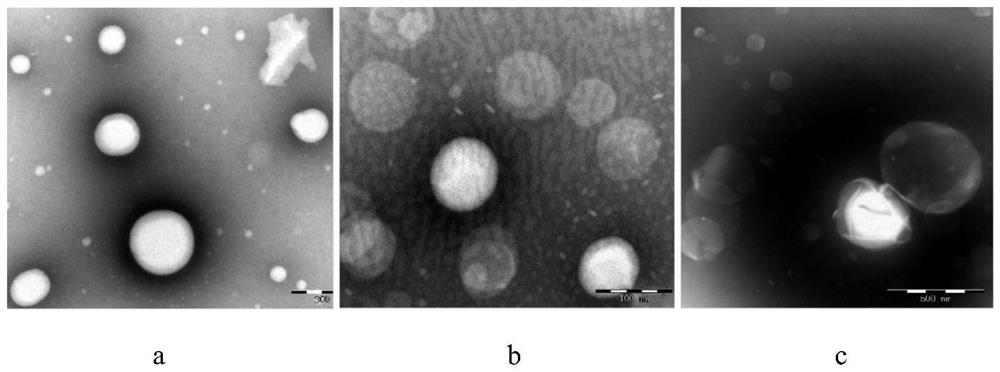
Image
Examples
Embodiment 1
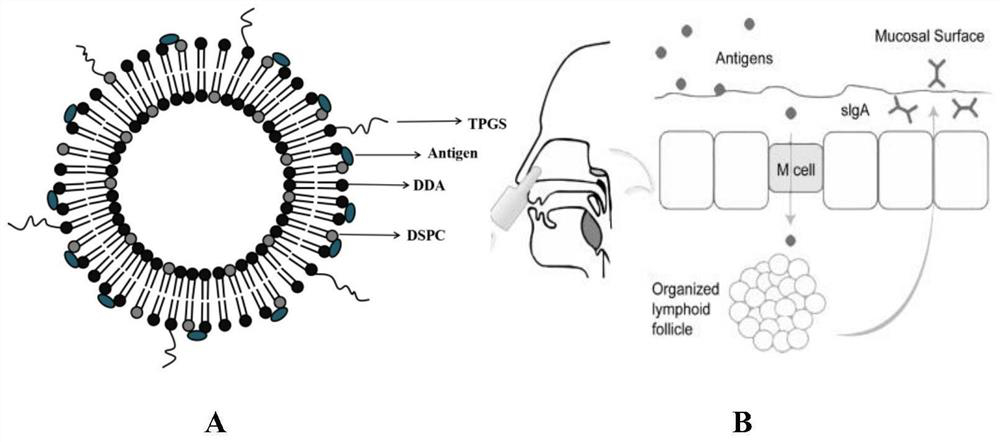
[0048] Embodiment 1: film dispersion method prepares DDA-DSPC-PEG cationic liposome influenza vaccine
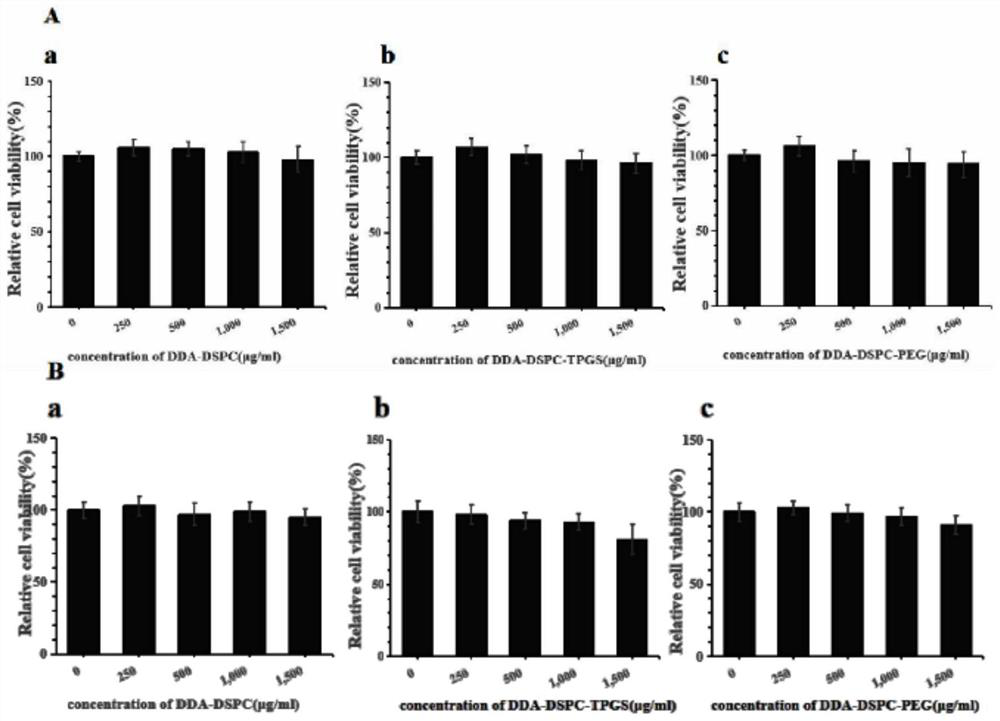
[0049] Precisely weigh cationic liposomes (dimethyl dioctadecyl ammonium DDA) and immunomodulators (distearoyl phosphatidylcholine DSPC, distearoyl phosphatidylethanolamine-polyethylene glycol 2000DSPE) of the prescription amount -PEG2000), above-mentioned DDA-DSPC-PEG molar ratio 4:15:1, is placed in 50ml round bottom flask, adds 2ml chloroform-methanol solution to dissolve (V 氯仿 :V 甲醇 =9:1), vortexed for 1 min and then evaporated in a constant temperature water bath at 37°C for 20 min on a rotary evaporator. After the organic solvent was evaporated, a lipid film was visible at the bottom of the round bottom flask. At this time, nitrogen gas was filled for 2 minutes to remove the residual organic solvent.
[0050] Then add a certain amount of Tris buffer solution (concentration: 10mmol / L, pH7.4) into the round bottom flask, and hydrate in a 65°C water bath for 40min to pr...
Embodiment 2
[0053] Embodiment 2: film dispersion method prepares DDA-DSPC cationic liposome influenza vaccine
[0054] Precisely weigh cationic liposomes (dimethyl dioctadecyl ammonium DDA) and immunomodulator (distearoylphosphatidylcholine DSPC) of prescription quantity, above-mentioned DDA-DSPC molar ratio 1:4, place in In a 50ml round bottom flask, add 2ml of chloroform-methanol solution to dissolve (V 氯仿 :V 甲醇 =9:1), vortexed for 1 min and then evaporated in a constant temperature water bath at 37°C for 40 min on a rotary evaporator. After the organic solvent was evaporated, a lipid film was visible at the bottom of the round bottom flask. At this time, nitrogen gas was filled for 2 minutes to remove the residual organic solvent.
[0055] Add a certain amount of Tris buffer solution (concentration is 5mmol / L, pH7.4) , and hydrated in a water bath at 65°C for 20 minutes to prepare DDA-DSPC blank liposomes.
[0056] Add the influenza vaccine stock solution to the DDA-DSPC blank lip...
Embodiment 3
[0058] Embodiment 3: film dispersion method prepares DDA-DSPC-TPGS cationic liposome influenza vaccine
[0059] Precisely weigh cationic liposomes (dimethyl dioctadecyl ammonium DDA) and immunomodulators (distearoylphosphatidylcholine DSPC, vitamin E polyethylene glycol succinate TPGS) of prescription quantity, above-mentioned DDA-DSPC-TPGS molar ratio is 4:15:1, is placed in 50ml round bottom flask, adds 2ml chloroform-methanol solution to dissolve (V 氯仿 :V 甲醇 =9:1), vortexed for 1 min and then evaporated in a constant temperature water bath at 37°C for 20 min on a rotary evaporator. After the organic solvent was evaporated, a lipid film was visible at the bottom of the round bottom flask. At this time, nitrogen gas was filled for 2 minutes to remove the residual organic solvent.
[0060] Then add a certain amount of PBS buffer solution (concentration: 30mmol / L, pH7.4) into the round bottom flask, and hydrate in a 65°C water bath for 20min to prepare DDA-DSPC-TPGS blank lip...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com