Ultrasonic response type polymer and nanoparticles, preparation method and application thereof
A nanoparticle and polymer technology, used in microcapsules, nanocapsules, pharmaceutical formulations, etc., can solve the problems of inflammatory response, large particle size, and unstable liposomes in the body, and achieve sensitive ultrasonic response and small dispersion coefficient. , the effect of low cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Embodiment 1 synthetic tetrahydropyranyl methacrylate monomer:
[0074] Firstly, 2-tetrahydropyranyl methacrylate monomer (Tetrahydropyranyl methacrylate, THPMA) was synthesized. 2-tetrahydropyran was synthesized from methacrylic acid (MAA) and 3,4-dihydro-2H-pyran (3,4-Dihydro-2H-pyran, DHP) by addition reaction p-Toluenesulfonic acid monohydrate (p-Toluenesulfonic acid monohydrate, TsOH) is added in the reaction system as the catalyst of reaction; Pyridine (Pyridine, Py) is added in the reaction system to make MAA and DHP An addition reaction occurs to produce the THPMA product.
[0075] TsOH, MAA, and Py were weighed and put into a 500mL round bottom flask according to the amount and ratio of each reactant designed in advance, as shown in Table 1, and 160mL of dichloromethane was added as a solvent, and then The measured DHP was slowly and gradually added dropwise to the reaction system at room temperature, and finally the stirring was turned on at a speed of 300 r...
Embodiment 2
[0081] Example 2 Synthesis and kinetics of PDMA-RAFT (poly N, N'-dimethylacrylamide-RAFT macromolecular chain transfer agent):
[0082] RAFT polymerization can obtain a linear polymer with a small dispersion coefficient of the polymer. In the present invention, all polymers are synthesized by RAFT polymerization.
[0083] First, adopt the method for RAFT polymerization, RAFT chain transfer agent (Chain Transfer Agent, CTA) 4-cyano-4-[(dodecylsulfanylthiocarbonyl) sulfanyl] pentanoic acid ([(dodecylsulfanylthiocarbonyl) )sulfanyl]pentanoic acid, CDSPA) and N,N'-dimethylacrylamide monomer (N,N'-Dimethylacrylamide, DMA) are prepared by copolymerizing the two into PDMA-RAFT (polyN, N'-dimethylacrylamide-RAFT macromolecular chain transfer agent), the synthetic route is as follows, that is, CDSPA and DMA are dispersed in 1,4-dioxane, and then the initiator AIBN is added, at 80°C A copolymerization reaction was carried out to obtain PDMA-RAFT. The proportions of each reaction are s...
Embodiment 3
[0092] The synthesis of embodiment 3 PDMA-co-PTHPMA:
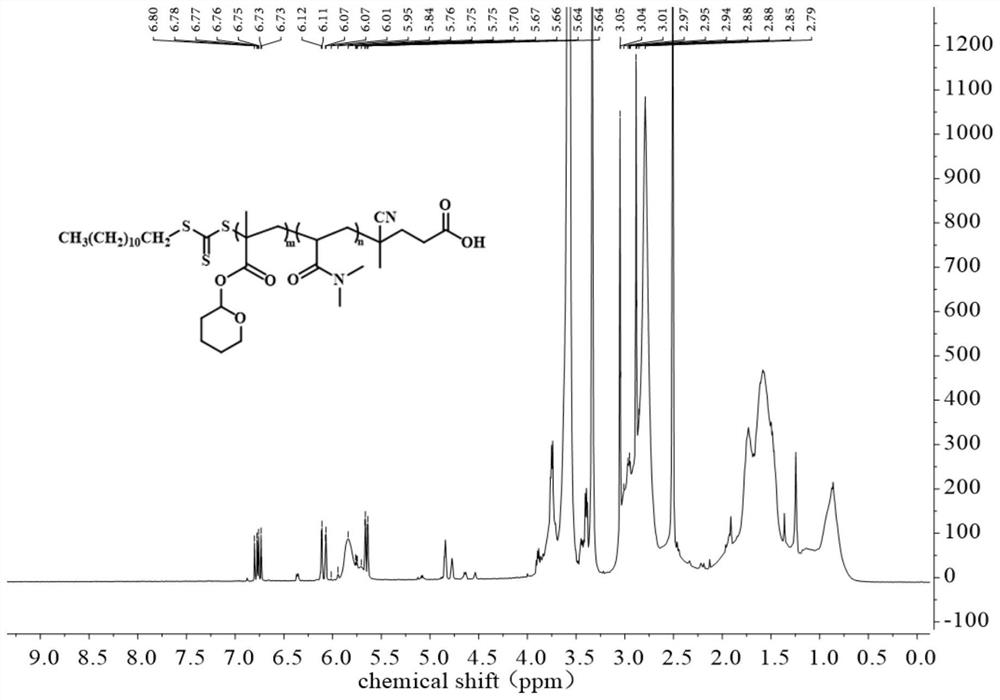
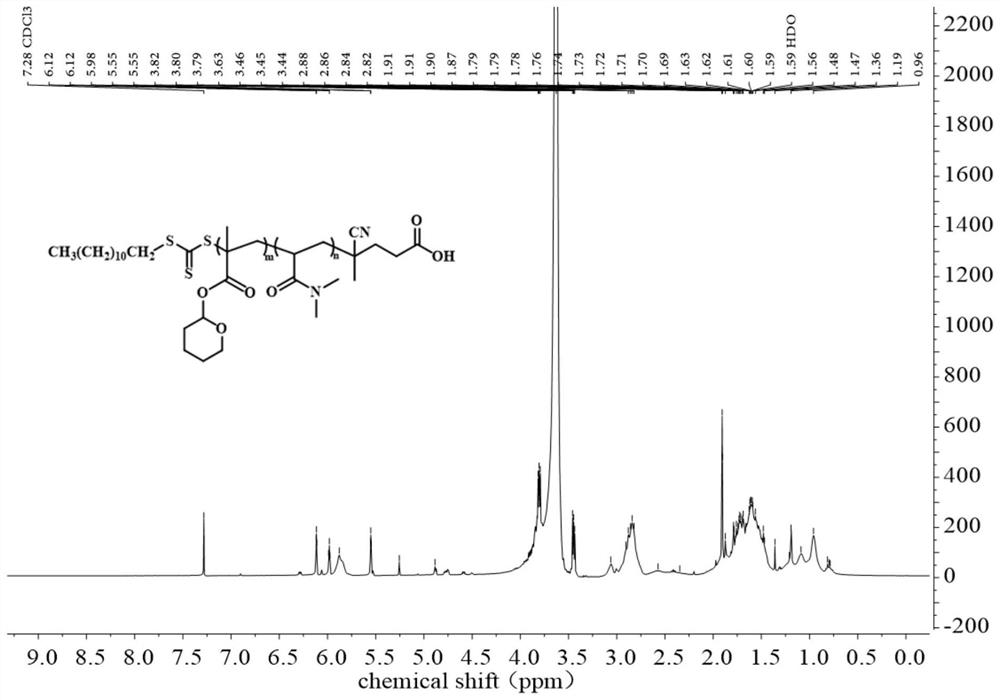
[0093] On the basis of the high-purity ultrasonic response compound THPMA monomer obtained in Example 1, THPMA monomer and N,N',-dimethylacrylamide monomer (N,N'- Dimethylacrylamide, DMA) adopts the method of RAFT polymerization, uses CDSPA chain transfer agent, and AIBN is used as initiator, and the two are prepared by copolymerization into PDMA-co-PTHPMA (poly(N,N'-dimethylacrylamide-co-tetrafluoroethylene Hydropyranyl methacrylate)), the specific reaction equation is shown in the following synthetic route. Subsequent ultrasonic response tests of THPMA and PDMA-co-PTHPMA polymers were carried out separately.
[0094] The synthetic route is as follows:
[0095]
[0096] In the process of synthesizing PDMA-co-PTHPMA using the RAFT polymerization method, according to the proportion of each component calculated in advance, as shown in Table 3, since the purchased DMA contains the polymerization inhibitor p-methoxyphenol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com