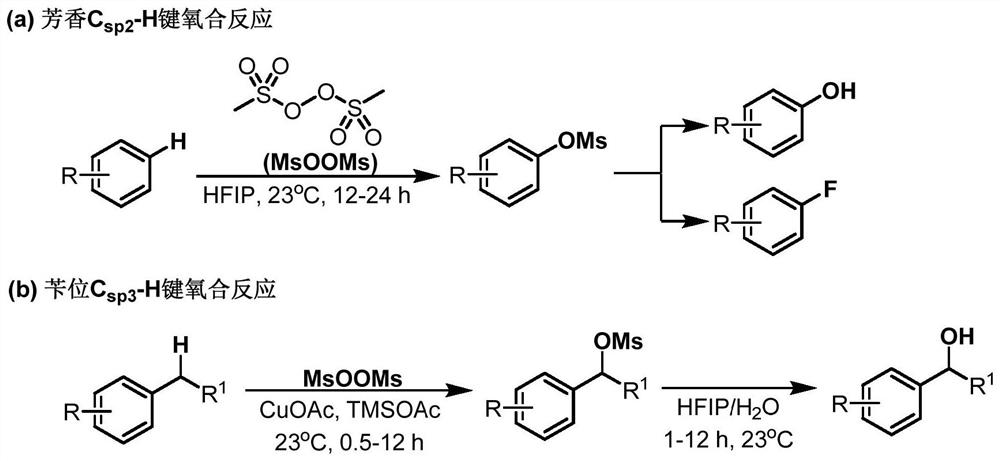
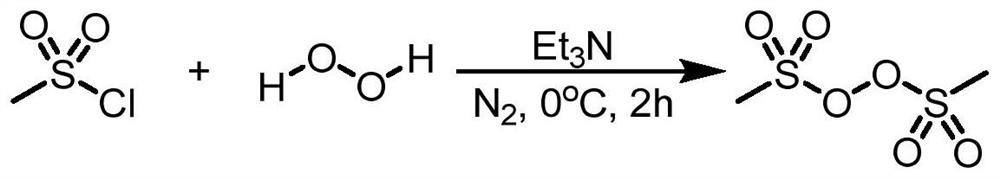
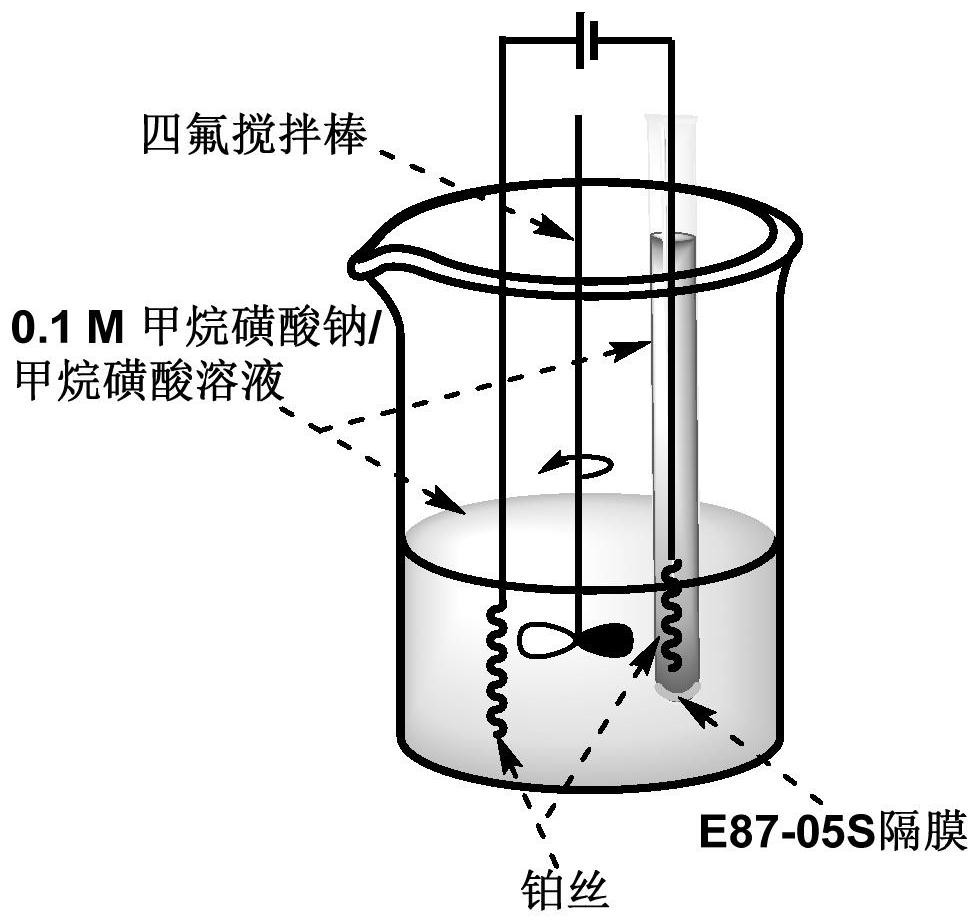
Electro-synthesis method of bis(methanesulfonyl)peroxide
A technology of peroxide and sulfonyl, applied in the field of electrochemical preparation of double peroxide, can solve the problems of reducing the reaction rate and catalytic efficiency, existing safety hazards, low Faradaic efficiency, etc., to improve the electrochemical reaction rate, prevent Safety incidents occur and the effect of improving Faraday's efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] In the second step, 1.6 g of sodium hydroxide was added to 40 mL of methanesulfonic acid to obtain a sodium methanesulfonate / methanesulfonic acid electrolyte with a concentration of about 1.0 mol / L. In the third step of the electrolysis process, the electrolysis temperature is set to 25°C, a direct current of 5.0mA is passed through both ends of the electrolysis tank, and the electrolysis time is set to 5.0h. In the fourth step, the yield was 86%
Embodiment 2
[0044] In the second step, 1.6 g of sodium hydroxide was added to 40 mL of methanesulfonic acid to obtain a sodium methanesulfonate / methanesulfonic acid electrolyte with a concentration of about 1.0 mol / L. In the third step of the electrolysis process, the electrolysis temperature is set to 35° C., a direct current of 5.0 mA is passed through both ends of the electrolysis cell, and the electrolysis time is set to 5.0 h. In the fourth step, the yield was 89%
Embodiment 3
[0046] In the second step, 1.6 g of sodium hydroxide was added to 40 mL of methanesulfonic acid to obtain a sodium methanesulfonate / methanesulfonic acid electrolyte with a concentration of about 1.0 mol / L. In the third step of the electrolysis process, the electrolysis temperature is set to 25°C, a direct current of 15.0mA is passed through both ends of the electrolysis cell, and the electrolysis time is set to 5.0h. In the fourth step, the yield was 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com