Chiral carboxylic acid compound as well as synthesis method and application thereof
A chiral carboxylic acid and compound technology, applied in the field of organic synthesis, can solve the problem of less chiral carboxylic acid and achieve high yield and enantioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
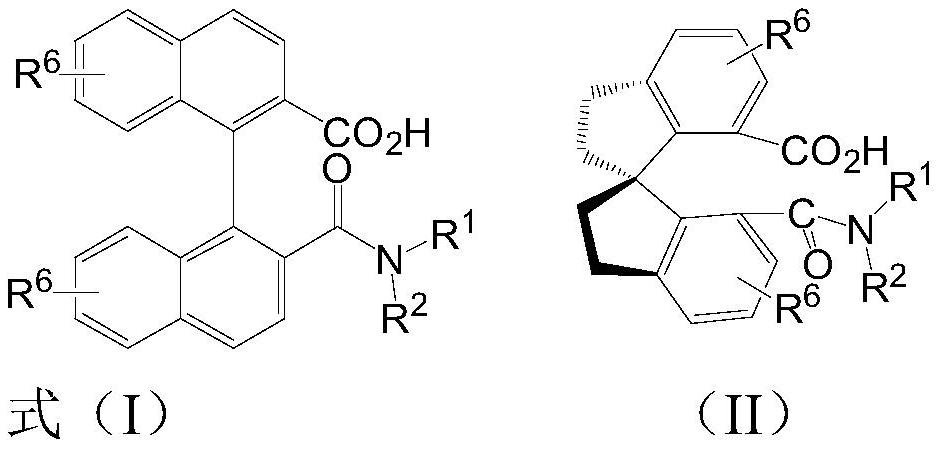
[0029] The preparation of embodiment 1 (S)-2'-(diisopropylcarbamoyl)-[1,1'-binaphthyl]-2-carboxylic acid:
[0030]
[0031] In the first step, 2.5 mmol of (S)-[1,1'-binaphthalene]-2,2'-dicarboxylic acid and 1.25 mmol of silver carbonate were mixed in 20 mL of organic solvent acetone, and 7.5 mmol of methyl iodide Add dropwise to the above reaction solution, the dropping temperature is 40°C, react at 40°C for 6 hours after dropping, after the reaction is complete, add ethyl acetate, filter, the filtrate is concentrated to dryness under reduced pressure, and the monoester compound B is obtained by column chromatography .
[0032] In the second step, the product obtained in the first step (2.2 mmoles of product B) was added to 20 mL of organic solvent dichloromethane, 5 drops of N,N'-dimethylformamide was added dropwise, and 3.3 mmoles of oxalyl chloride was added dropwise to To the above reaction solution, the dropwise addition temperature was 0°C, and after the dropwise rea...
Embodiment 2
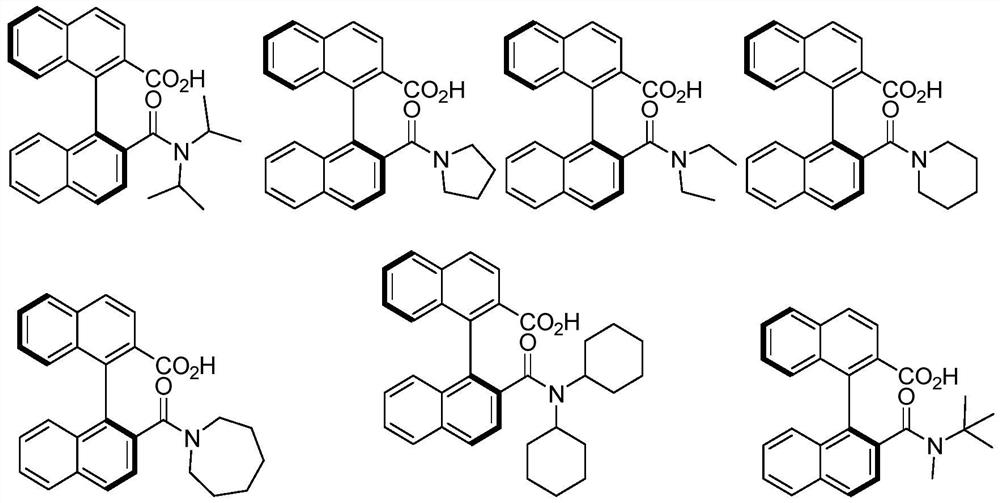
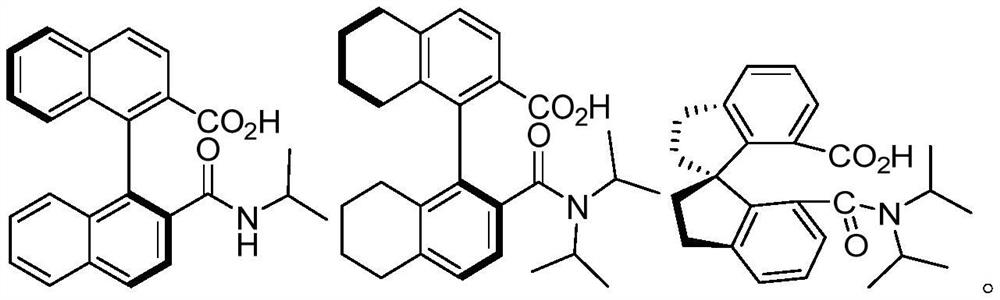
[0035] Embodiment 2 Preparation of other various chiral carboxylic acids
[0036] The preparation process is the same as in Example 1, but the amine added in the third step is replaced by other correspondingly substituted amines to obtain the corresponding chiral carboxylic acid data as follows:
[0037] (S)-2'-(pyrrolidine-1-carbonyl)-[1,1'-binaphthyl]-2-carboxylic acid
[0038]
[0039] 1 H NMR (400MHz, CDCl 3 )δ14.61(s,1H),8.04(dd,J=8.5,2.8Hz,2H),7.94(t,J=9.0Hz,2H),7.83(d,J=8.5Hz,1H),7.56– 7.44(m,3H),7.31(dd,J=8.4,7.0Hz,1H),7.28(s,1H),7.12(d,J=8.5Hz,1H),7.01(d,J=8.6Hz,1H ),3.61–3.55(m,1H),3.43–3.30(m,2H),3.10–3.03(m,1H),1.92–1.74(m,2H),1.69–1.63(m,1H),1.47–1.35 (m,1H). 13 C NMR (100MHz, CDCl 3 ) Δ171.2,170.9,135.0,134.1,134.0,133.9,132.5,132.0, 139.5, 129.3,128.2,127.9,126.8,125.7,25.7,25.7,25.7,25.7,25.7,25.3,3.3,3.3,3.3,3.3,3.3,3.3,3.3,3.3,3.3. , 24.1. HRMS (ESI) calcd for C 26 h 20 NO 3 - (M-H) - :394.1449,found:394.1446.
[0040] (S)-2'-(Diethylcarbamoyl...
Embodiment 3
[0064] Example 3 Chiral carboxylic acid was used as a ligand for ruthenium-catalyzed cyclization reaction of sulfoximine derivative 1a with α-carbonylsulfide ylide derivative 2a to prepare chiral sulfoximine derivative 3aa.
[0065] 0.1 mmol of sulfoximine derivative 1a, 0.1 mmol of α-carbonylsulfide ylide derivative 2a, 0.0025 mmol of p-cymene ruthenium(II) dichloride dimer, 0.02 mmol of hexa Silver fluoroantimonate, 0.01 mmol of (S)-2'-(diisopropylcarbamoyl)-[1,1'-binaphthyl]-2-carboxylic acid mixed in 2 ml of 1,2-dichloroethane In alkanes, the reaction was carried out at 35° C. for 12 hours under the protection of nitrogen. After the reaction, the product chiral sulfoximine derivative 3aa was obtained by thin-layer silica gel plate chromatography.
[0066]
[0067] Characterization of typical product 3aa:
[0068] (S)-1,3-Diphenylbenzo[e][1,2]thiazine 1-oxide
[0069]
[0070] Yield 96%, ee 98%. HPLC [AD-H] (n-hexane / isopropanol=80 / 20, 1.2 ml / min) λ=254nm, tr=13.9m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com