Cyano quinoxaline red light thermal excitation delayed fluorescence material, synthesis method and application thereof
A technology of cyanoquinoxalines and delayed fluorescence, which is applied in the field of electroluminescent materials, can solve the problems of enhanced intermolecular interaction, concentration quenching, and increased polarity, and achieve the purpose of inhibiting intermolecular interaction and inhibiting quenching. The effect of destroying and improving the transmission ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0081] In the present invention, a preparation method of the cyanoquinoxaline-based red-light thermally excited delayed fluorescence material is also provided, and the material is prepared from raw materials including halogenated aromatic ketone compounds and diaminophthalonitrile compounds , preferably, the method comprises the following steps:
[0082] Step 1. Add the halogenated aromatic ketone compound and the reactant I into the solvent, stir and react to obtain the intermediate I.
[0083] The halogenated aromatic ketones are selected from halogenated phenyl monoketones or halogenated phenyl diketones, preferably 1-halogenated phenyl-2-halogenated alkan-1-ones or halogenated phenyl- 1,2-Diketone, more preferably 1-halophenyl-2-haloethan-1-one or halophenylethanedione, such as 2-bromo-1-(4-bromophenyl)ethyl -1-one, 1,2-bis(4-bromophenyl)ethane-1,2-dione.
[0084] The reactant I is a diaminophthalonitrile compound or an aromatic amine compound I. When the reactant I is ...
Embodiment 1
[0184] 10mmol of 4,5-diaminophthalonitrile, 10mmol of 2-bromo-1-(4-bromophenyl)ethan-1-one, 50ml of water and 0.9g of hexadecyltrimethyl ammonium bromide, stirred and reacted at 100°C for 12 hours, then poured into ice water, filtered with suction, and washed the resulting solid with absolute ethanol to obtain 2-(4-bromophenyl)quinoxaline-6,7- Dicarbonitrile.
[0185] Mix 2mmol 2-(4-bromophenyl)quinoxaline-6,7-dicarbonitrile with 2.5mmol diphenylamine, 0.06mmol tridibenzylideneacetone dipalladium, 6mmol cesium carbonate, 0.3mmol tri-tert-butylphosphine Mix, add 10mL xylene as a solvent, reflux reaction at 135°C for 12h, and then pour into ice water. Extract with water and dichloromethane (the volume ratio of the two is 1:1), combine the organic layers, and remove the organic solvent after drying to obtain a crude product, which is purified by column chromatography with a mixed solvent of petroleum ether and dichloromethane as eluent (The volume ratio of the two is 1:2), to o...
Embodiment 2
[0204] According to the synthesis method of compound 1 in Example 1, compound 2 was prepared. The only difference is: 4,5-diaminophthalonitrile is replaced by 2,3-diaminoterephthalonitrile.
[0205] The compound 2 that obtains is carried out proton nuclear magnetic resonance spectrum analysis, test data is: 1 H NMR (TMS, CDCl 3 ,400MHz): δ=9.54(s,1H),8.24(d,J=8.9Hz,2H),8.16(d,J=7.6Hz,1H),8.07(d,J=7.6Hz,1H),7.37 (t,J=7.9Hz,4H),7.25–7.21(m,4H),7.21–7.16(m,4H).
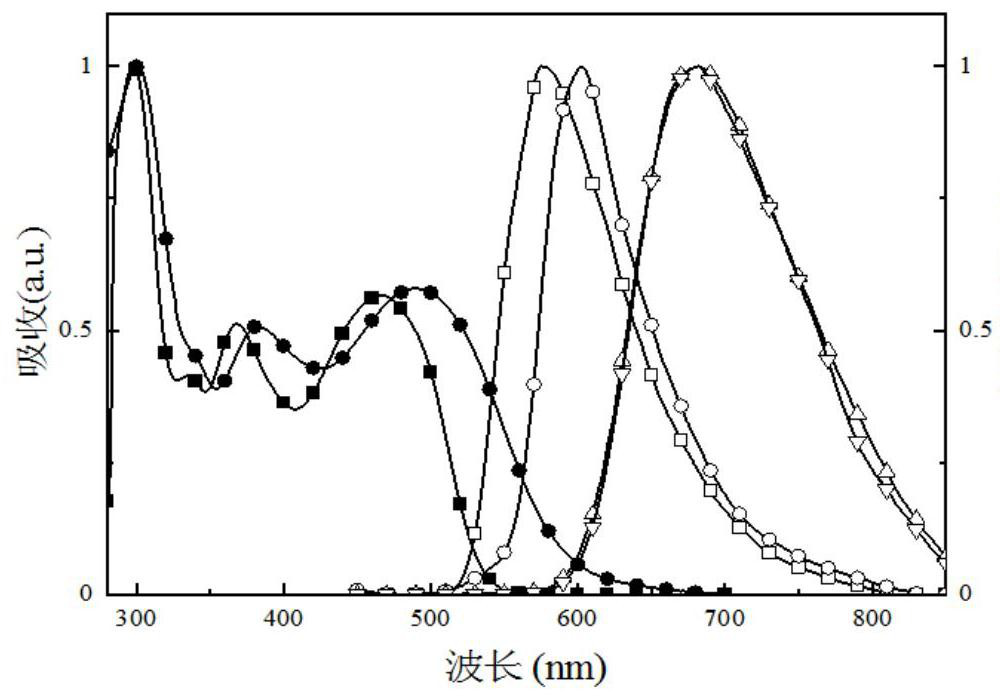
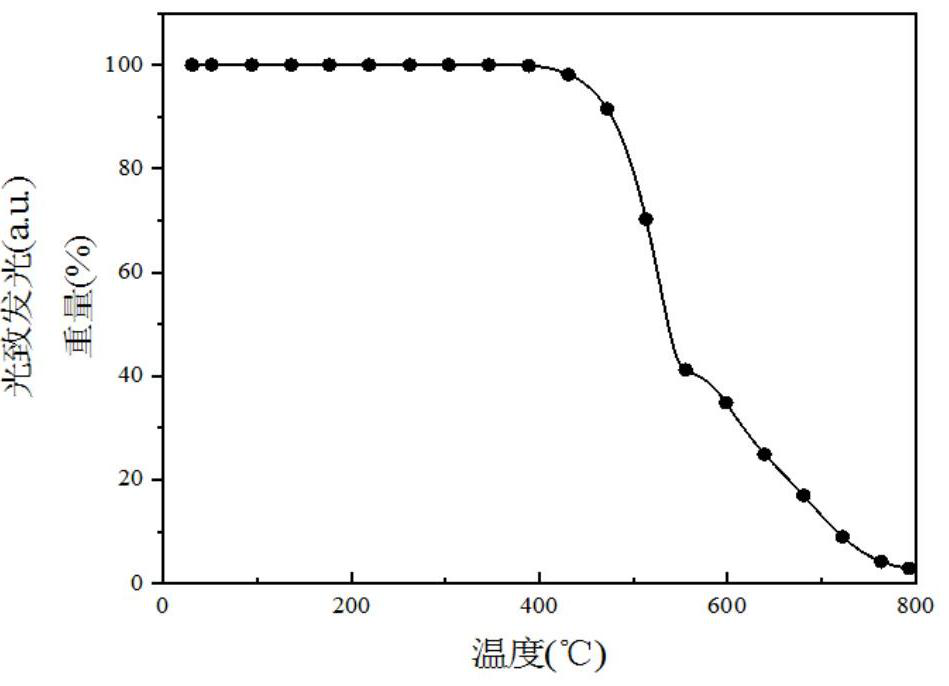
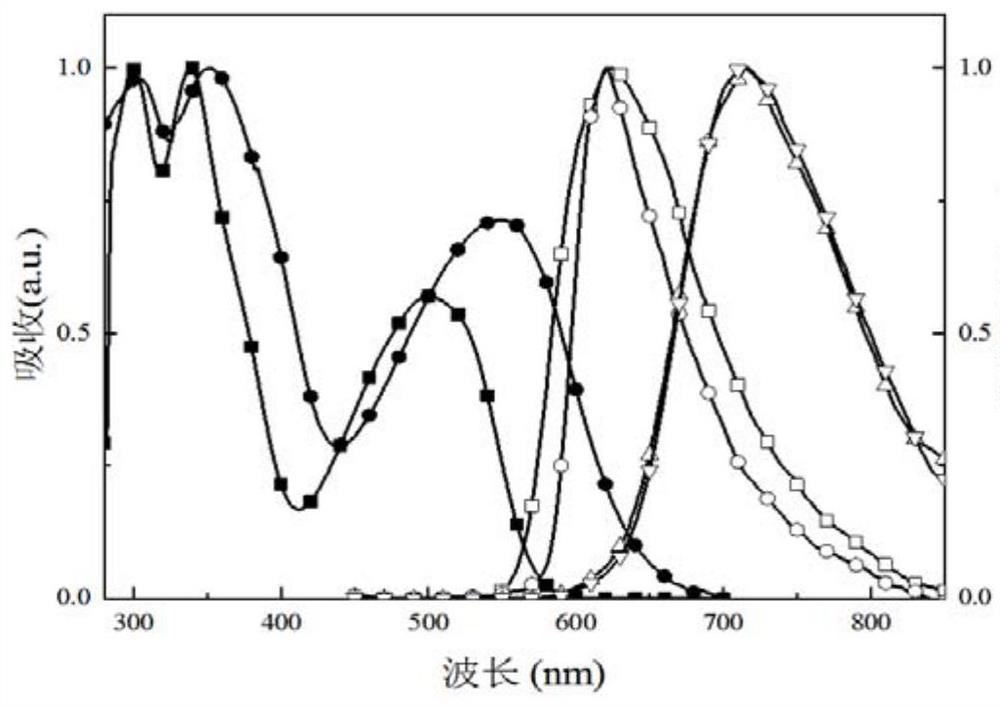
[0206] The obtained compound IV was subjected to thermogravimetric analysis, and the test data were as follows: Figure 4 shown by Figure 4 It was found that the cracking temperature of the obtained compound was 422°C.
[0207] According to the preparation method of the electroluminescent red light device in Example 1, the mixture of compound 2 and CBP (wherein the mass fraction of compound 2 is 20%) was used as the light-emitting layer material to prepare the electroluminescent red light device.
[0208] The stru...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com