Phenothiazine-containing pillararene fluorescent adsorption material and preparation method thereof
An adsorption material, phenothiazine technology, applied in the field of phenothiazine-containing columnarene fluorescent adsorption material and its preparation, can solve the difficulty of purification and separation, limit the research and application of columnarene and its derivatives, and it is difficult to obtain compact and rigid crystals and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0051] The preparation method of compound 6 comprises the following steps:
[0052](1) Compound 1 (21.0 g, 0.15 mol) (1,4-dimethoxybenzene, purchased from Adamas, specification 500 g, purity 99%) was dissolved in dichloroethane (600 mL) at room temperature, Add paraformaldehyde (9.0g, 0.30mmol) and trifluoroacetic acid (30mL) to the solution, and stir at reflux for 5h to obtain compound 2; wherein, the volume ratio of dichloromethane and trifluoroacetic acid is 20:1, compound 1 and poly The molar ratio of polyoxymethylene is 1:2.
[0053] (2) At room temperature, compound 2 (7.000g, 9.30mmol) was dissolved in a mixture of dichloromethane (350mL) and tetrahydrofuran (350mL), and ceric ammonium nitrate (11.235g, 20.5mmol) was added dropwise to the solution ) aqueous solution (42mL), after dropping, stirred for 24h to obtain compound 3; wherein, the volume ratio of dichloromethane and tetrahydrofuran was 1:1, and the molar ratio of compound 2 and ceric ammonium nitrate was 1:2.2...
Embodiment 1
[0058]
[0059] In the glove box, under argon atmosphere, compound 6 (414mg, 0.5mmol), sodium tert-butoxide (170mg, 1.5mmol), palladium acetate (5mg, 0.01mmol), tri-tert-butylphosphine tetrafluoroborate (12mg, 0.02mmol), 2-bromo-9-fluorenone (259mg, 1.0mmol) and toluene (3mL) were mixed at a temperature of 100-110°C and stirred for 23h under an argon atmosphere. The reaction solution was cooled to room temperature, 50 mL of water was added, extracted with dichloromethane (50 mL×3), the organic phases were combined, dried over sodium sulfate, and purified by column chromatography to obtain 327 mg of compound 7 with a yield of 65.0%.
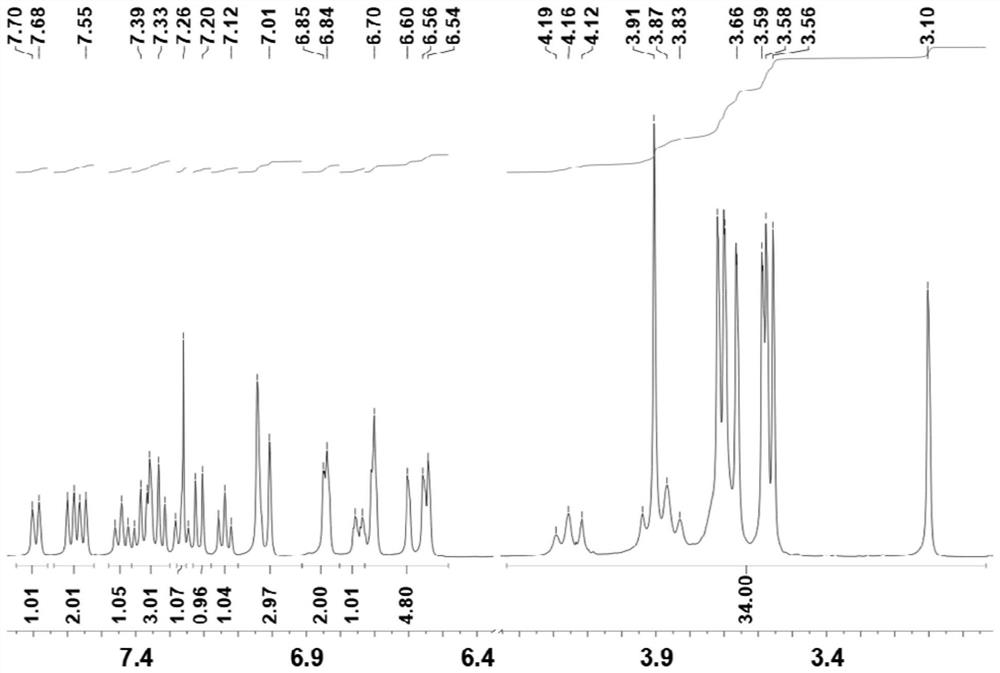
[0060] figure 1 Compound 7 prepared for Example 1 of the present invention 1 H-NMR diagram, 1 H NMR (400MHz, CDCl 3 )δ=7.69(d, J=8.0Hz, 1H), 7.59(d, J=8.0Hz, 1H), 7.56(d, J=4.0Hz, 1H), 7.44(t, J=8.0Hz, 1H) ,7.32-7.40(m,3H),7.27(t,J=6.0Hz,1H),7.21(d,J=6.0Hz,1H),7.14(t,J=8.0Hz,1H),7.04(s, 2H), 7.01(s, 1H), 6.85(m, 2H), 6.75(m, 1H), 6.54-6.70...
Embodiment 2
[0065]
[0066] In the glove box, under argon atmosphere, compound 6 (455.4mg, 0.55mmol), sodium tert-butoxide (187mg, 1.65mmol), palladium acetate (5mg, 0.01mmol), tri-tert-butylphosphine tetrafluoroboric acid Salt (12mg, 0.02mmol), 2-bromoanthracene (257mg, 1.0mmol) and toluene (3.2mL) were mixed at 100-110°C and stirred for 22h under an argon atmosphere. The reaction solution was cooled to room temperature, 50 mL of water was added, extracted with dichloromethane (50 mL×3), the organic phases were combined, dried over sodium sulfate, and purified by column chromatography to obtain 338 mg of compound 9 with a yield of 61.2%.
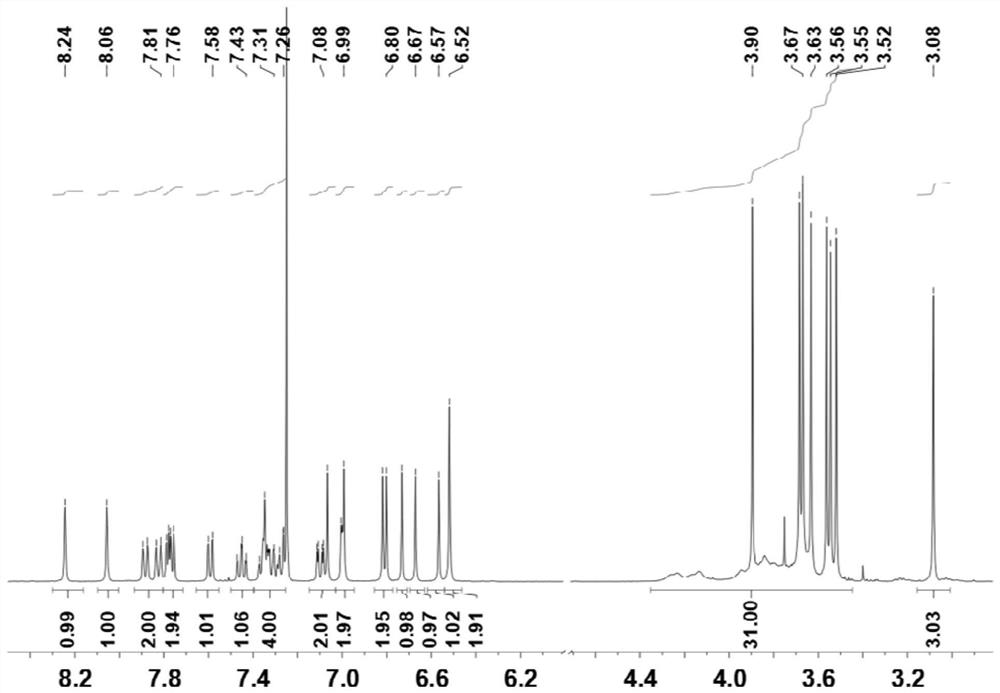
[0067] image 3 For the compound 9 prepared by the embodiment of the present invention 2 1 H-NMR diagram, 1 H NMR (400MHz, CDCl 3 )δ=8.24(s,1H),8.06(s,1H),7.88(d,J=8.0Hz,1H),7.82(d,J=8.0Hz,1H),7.79(d,J=4.0Hz, 1H), 7.77(d, J=4.0Hz, 1H), 7.59(d, J=8.0Hz, 1H), 7.45(t, J=8.0Hz, 1H), 7.34-6.24(m, 4H), 7.10( d,J=4.0Hz,J=8.0Hz,1H),7.07(s,1H),7.00(d,J=...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com