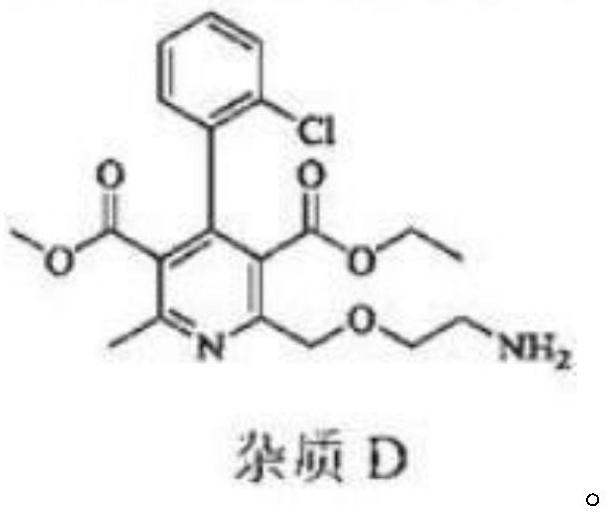
Levoamlodipine besylate tablet and preparation method thereof
A technology of levamlodipine besylate and levorotatory besylate, which is applied in the field of levamlodipine besylate tablets and its preparation, to achieve the effects of being easy to take, high yield, and inhibiting the production of impurity D
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1 A kind of preparation method of levamlodipine besylate
[0028] The present embodiment is a preparation method of levamlodipine besylate, and the specific preparation process comprises the following steps:
[0029] 1) Preparation of S-(-)-amlodipine-R-(-)-mandelate
[0030] Take 950 mL of N,N-dimethylformamide and 150 mL of water (the volume ratio of N,N-dimethylformamide to water is BL1=6.33:1), stir and mix evenly, and filter to obtain a resolution aid solution for backup;
[0031] Take 20.7g of R-(-)-mandelic acid and add it to 200mL of resolution aid solution, stir to dissolve, filter to remove insoluble impurities, and obtain a resolution agent solution for subsequent use;
[0032] Take 100g of racemic amlodipine and add it to 700mL of resolution aid solution, stir to dissolve, filter to remove insoluble impurities, add resolution solution dropwise to the filtrate, stir and react at room temperature for 1h, add a trace amount of S-(-)-ammonia Seed c...
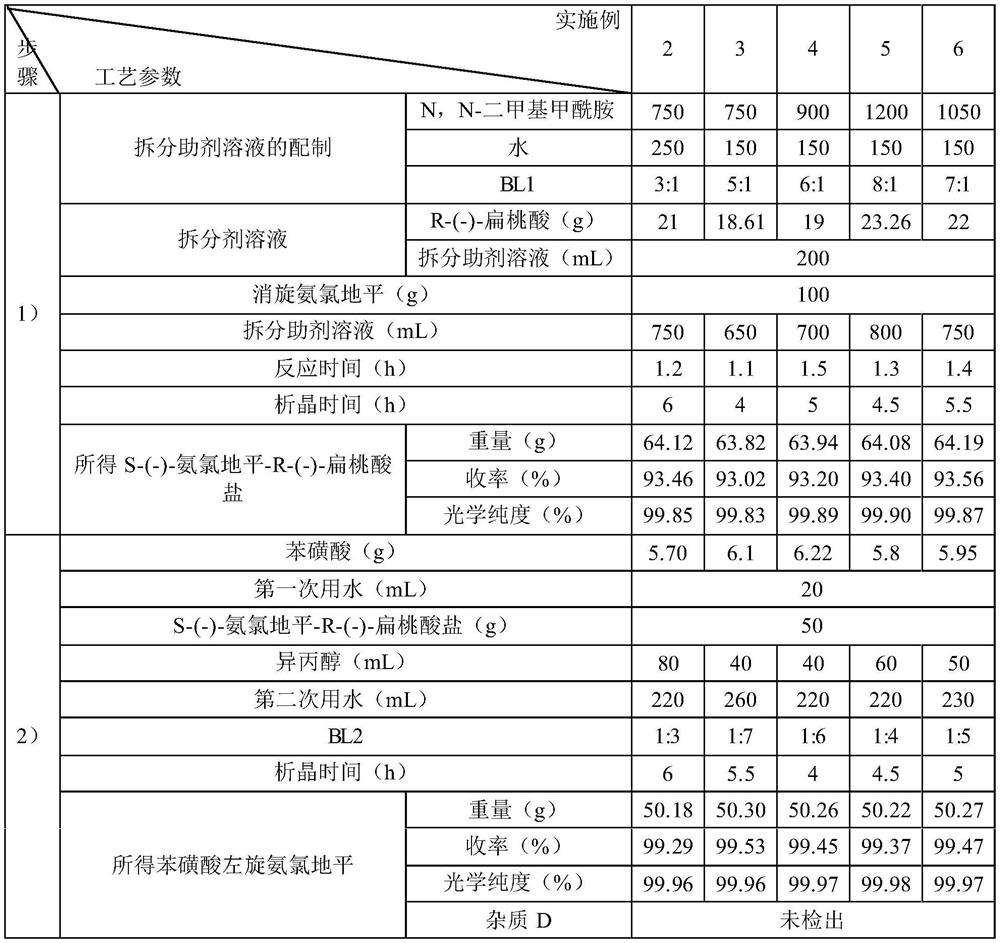
Embodiment 2~6
[0037] The preparation method of embodiment 2~6 levamlodipine besylate
[0038] Embodiments 2~6 are respectively a kind of preparation method of levamlodipine besylate, and their steps are basically identical with embodiment 1, and the difference is only in the difference of raw material consumption and process parameter, see Table 1 for details:
[0039] List of various process parameters in Table 1 Examples 2 to 6
[0040]
[0041] The contents of other parts of Examples 2 to 6 are the same as those of Example 1.
[0042] The levamlodipine besylate prepared in Examples 1-6 has high yield and good purity.
Embodiment 7
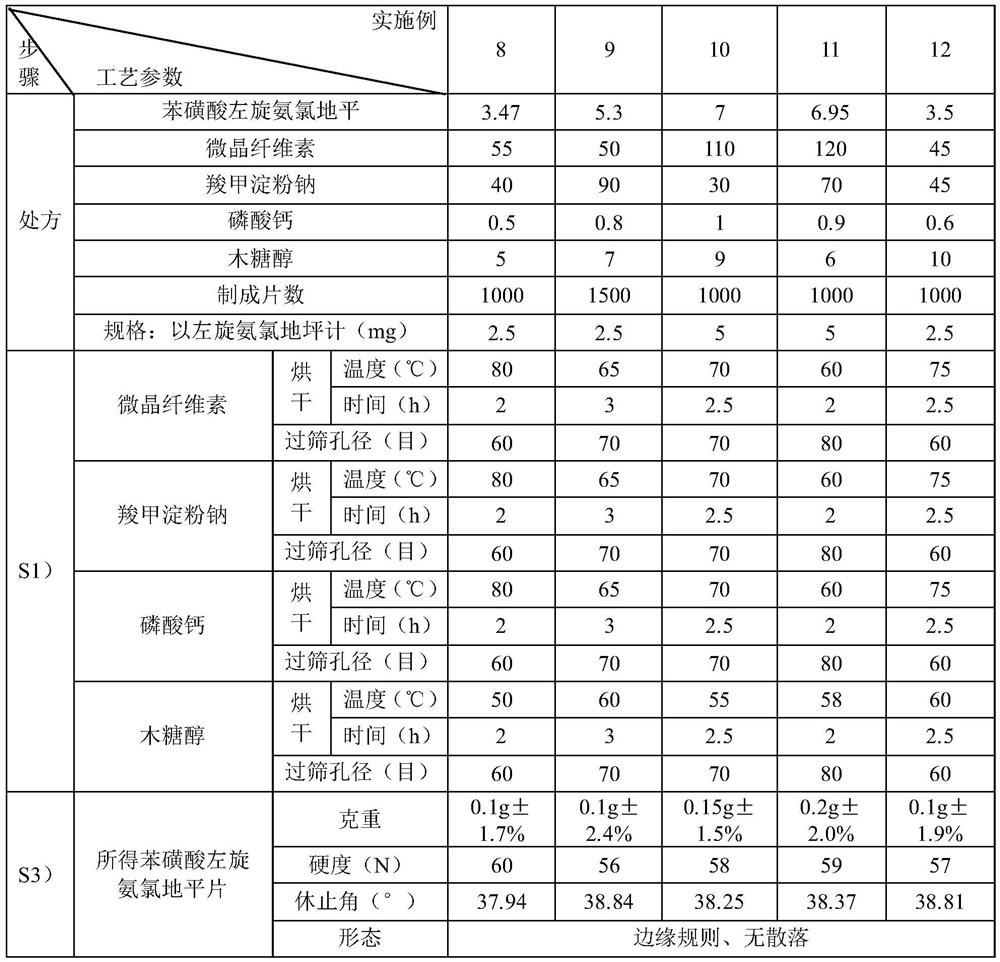
[0043] Embodiment 7 A kind of preparation method of levamlodipine besylate tablet
[0044] In the present embodiment, the prescription of levamlodipine besylate tablet is as follows:
[0045] Table 2 prescription (specification: 5mg in terms of levamlodipine)
[0046] Levoamlodipine besylate (from Example 1) 6.94g microcrystalline cellulose 100g Sodium starch glycolate 85g calcium phosphate 0.8g Xylitol 8g production 1000 pieces
[0047] In the present embodiment, the preparation method of levamlodipine besylate tablet comprises the following steps:
[0048] S1) drying microcrystalline cellulose, sodium starch glycolate, calcium phosphate and xylitol at 60°C for 2h respectively, passing through a 60-mesh sieve, for subsequent use;
[0049] S2) respectively take the microcrystalline cellulose, sodium starch glycolate, calcium phosphate and xylitol of the recipe quantity, adopt the equal amount incremental addition method, stir and mi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| angle of repose | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com