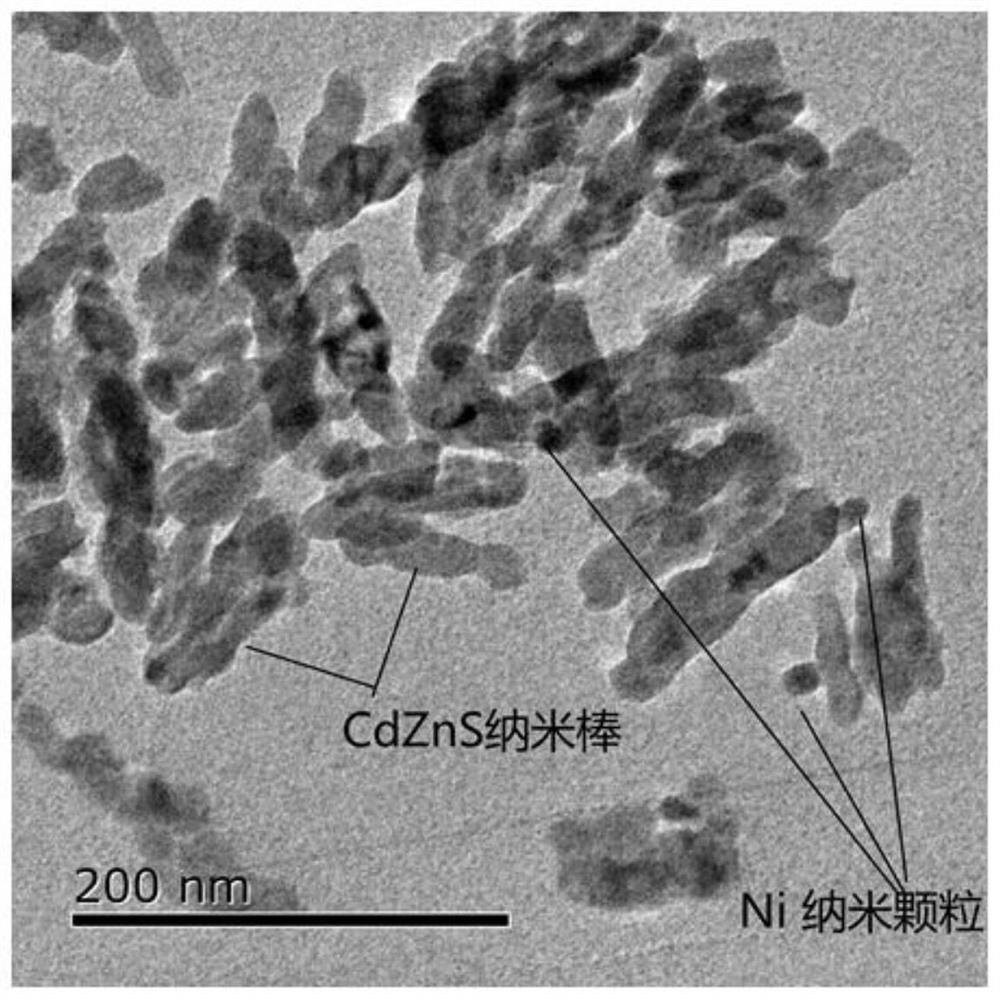
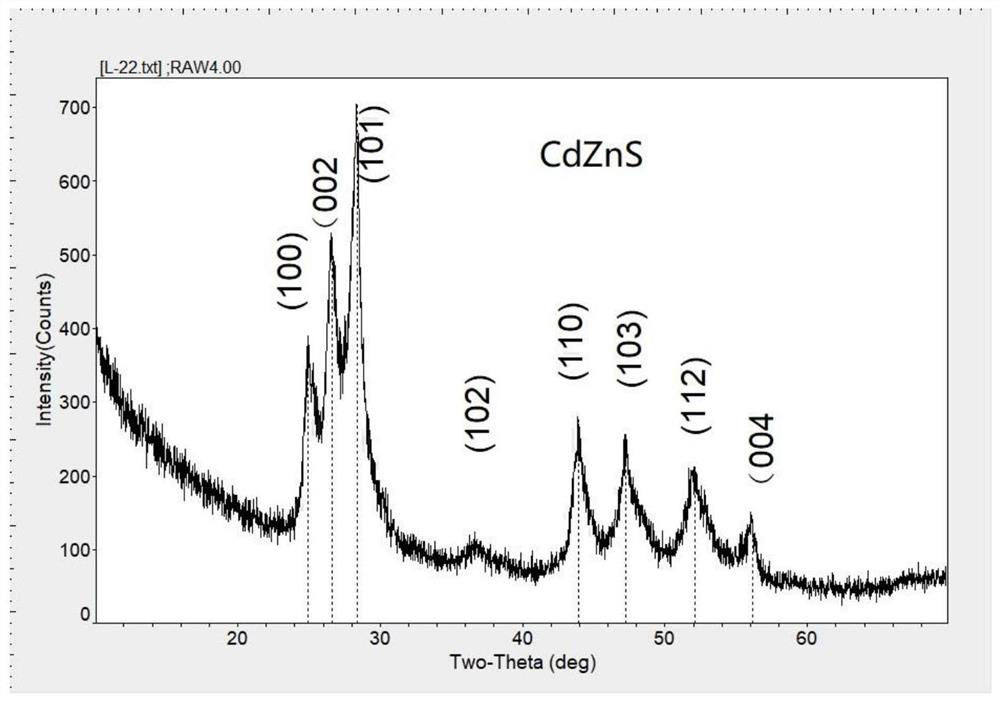
Zinc cadmium sulfide nanorod and nickel nanorod heterojunction photocatalyst, preparation method thereof, hydrogen production system and hydrogen production method
A technology of zinc cadmium sulfide and photocatalyst, which is applied in the direction of catalyst activation/preparation, physical/chemical process catalyst, chemical instrument and method, etc. It can solve the problems of easy photocorrosion, poor stability, easy recombination of photogenerated electrons and holes, etc. , to achieve the effects of improving hydrogen production efficiency, avoiding recombination, and improving migration performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] 1) Dissolve 2mmol cadmium chloride, 2mmol zinc chloride, 0.4g urea and 8mmol cysteine in a mixed solution composed of 70ml water and 7ml ethanolamine, stir and mix evenly, place the mixed solution in a hydrothermal reaction tank, Reacted at 160°C for 16 hours to obtain a yellow precipitate, which was filtered and washed three times with deionized water and ethanol respectively to obtain Zn 0.5 Cd 0.5 S nanorod photocatalyst.
[0052] 2) Add 0.5g Zn 0.5 Cd 0.5 S nanorods were ultrasonically dispersed in 100ml of distilled water, and then 53mg of nickel sulfate, 100mg of polyethylene glycol (molecular weight 2000), 0.2g of urea and 1ml of hydrazine hydrate were added. After stirring and mixing evenly, the solution was transferred to a 200mL reactor and reacted at 95°C for 12h. The product was separated by centrifugation and washed twice with deionized water and ethanol to obtain Zn0.5 Cd 0.5 S nanorod-metal nickel nano-heterojunction composite material, vacuum-drie...
Embodiment 2
[0055] 1) Dissolve 12mmol cadmium acetate, 8mmol zinc acetate, 0.6g urea and 50mmol cysteine in a mixed solution composed of 75ml water and 4ml ethanolamine, stir and mix evenly, place the mixed solution in a hydrothermal reaction tank, and heat it at 180 ℃ for 6 hours, a yellow precipitate was obtained, which was filtered and washed three times with deionized water and ethanol respectively to obtain Zn 0.4 Cd 0.6 S nanorod photocatalyst.
[0056] 2) Add 1.0g Zn 0.4 Cd 0.6 S nanorods were ultrasonically dispersed in 100ml of distilled water, and then 100mg of nickel sulfate, 200mg of polyethylene glycol (molecular weight 2000), 0.2g of urea and 1.5ml of hydrazine hydrate were added. After stirring and mixing evenly, the solution was transferred to a 200mL reactor and reacted at 85°C for 12h. The product was separated by centrifugation and washed twice with deionized water and ethanol to obtain Zn 0.4 Cd 0.6 S nanorod-metal nickel nano-heterojunction composite material,...
Embodiment 3
[0059] 1) Dissolve 8mmol cadmium nitrate, 12mmol zinc nitrate, 1.0g urea and 40mmol cysteine in a mixed solution composed of 70ml water and 7ml ethanolamine, stir and mix evenly, place the mixed solution in a hydrothermal reaction tank, and heat it at 170 ℃ for 8 hours, a yellow precipitate was obtained, which was filtered and washed three times with deionized water and ethanol respectively to obtain Zn 0.6 Cd 0.4 S nanorod photocatalyst.
[0060] 2) Add 1.0g Zn 0.6 Cd 0.4 S nanorods were ultrasonically dispersed in 100ml of distilled water, and then 300mg of nickel sulfate, 1.0g of polyethylene glycol (molecular weight 1000), 0.2g of urea and 10ml of hydrazine hydrate were added. After stirring and mixing evenly, the solution was transferred to a 200mL reactor and reacted at 100°C for 2h. The product was separated by centrifugation and washed twice with deionized water and ethanol to obtain Zn 0.6 Cd 0.4 S nanorod-metal nickel nano-heterojunction composite material, v...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com