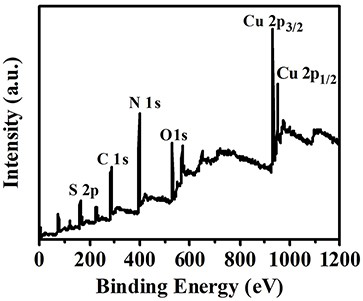
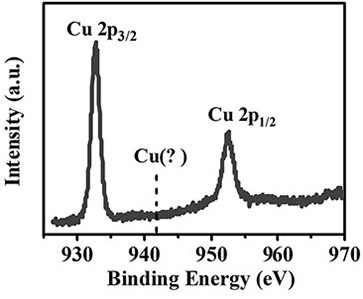
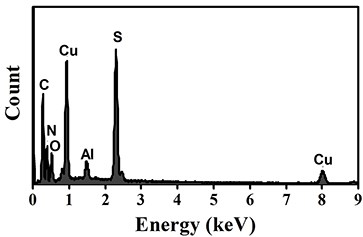
4, 6-diamino-2-mercaptopyrimidine copper (I) sensitizer capable of generating singlet oxygen
A technology of mercaptopyrimidine and singlet oxygen, which is applied in the field of synthesis of photoluminescent transition metal complex sensitized materials, can solve the problems of visible light sensitivity, time-consuming treatment, and is still in the initial stage, and achieves simple preparation methods, leaching Low efficiency and stable luminous effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Synthesis of Cu-dapy luminescent powder
[0026] Add 40 mL of 1.5 mmol·L to a 250 mL three-neck round bottom flask -1 An aqueous solution of cetyl ammonium bromide and 7.4 mg (0.03 mmol) of copper sulfate pentahydrate (CuSO 4 ·5H 2 O), stirred at room temperature until the copper source was completely dissolved, then added 4.2 mg (0.03 mmol) of 4,6-diaminomercapto-2-pyrimidine (dapyH) under nitrogen atmosphere, stirred vigorously for 30 minutes, and then added 10 mL , 3 mmol·L -1 Stannous chloride (SnCl 2 ) solution, and continued to stir for 4 hours. After fully reacting, the resulting solution was centrifuged (11000 rpm) for 10 min and washed three times with ultrapure water. Finally, the centrifuged precipitate was completely dried in a vacuum oven at 40°C to obtain the final pale yellow product Cu(dapy)·4H 2 O(Cu-dapy). The Cu-dapy solution used in subsequent experiments was obtained by redispersing the vacuum-dried powder in deionized water.
[00...
Embodiment 2
[0030] Example 2: Photoluminescence properties of Cu-dapy
[0031] Such as Figure 4 The fluorescence spectrogram of the suspension prepared by the Cu-dapy prepared in Example 1 with water as a solvent. Cu-dapy was dispersed with deionized water at room temperature to obtain a light yellow suspension (1mg.mL -1 ), which emits intense orange-yellow light under the irradiation of a 365 nm ultraviolet lamp. 368 nm was selected as the excitation wavelength, and Cu-dapy had the strongest emission peak at 580 nm. This strong orange-yellow light emission was caused by ligand-to-metal charge transfer (LMCT).
Embodiment 3
[0032] Embodiment 3: Luminescence stability of Cu-dapy under different pH conditions
[0033] Such as Figure 5 The relationship between the fluorescence intensity and the pH value of the Cu-dapy aqueous solution prepared in Example 1. In the range of pH = 2~7, Cu-dapy showed strong and stable fluorescence emission; in the environment of pH = 6~7, its fluorescence intensity reached the highest; when the pH increased to 8, the Cu-dapy The fluorescence intensity begins to drop sharply because in an alkaline environment, copper ions combine with hydroxide ions to form non-luminescent basic copper salts. The luminescent stability of Cu-dapy under neutral or acidic conditions lays the foundation for its application under physiological conditions.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com