Preparation method and application of vesicular phosphate ion functionalized cobalt oxide nano material
A nanomaterial, vesicle-like technology, applied in the field of electrocatalysis, can solve the problem that the charge energy storage and conversion ability cannot be fully released, achieve good electrochemical kinetic effect, improve electrocatalytic activity, and reduce overpotential. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The Co(NO 3 ) 2 . 6H 2 O was dissolved in deionized water to make a 0.5 mol / L cobalt salt aqueous solution.
[0041] will C 4 H 6 N 2 Dissolve in deionized water to make 1.095 mol / L organic ligand aqueous solution.
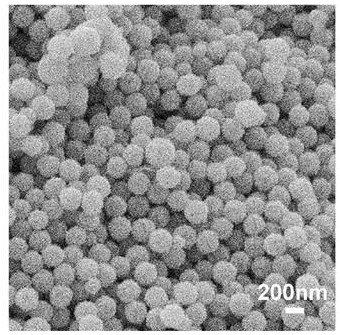
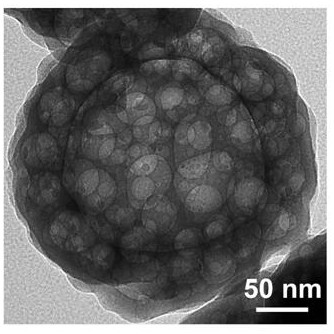
[0042] Dissolve 0.3 g of cetyltrimethylammonium bromide in 27 mL of deionized aqueous solution, and add 7.5 mL of the above-mentioned organic ligand aqueous solution and 375 μL of cobalt salt aqueous solution at 37 °C under the condition of constant temperature water bath stirring, and after stirring for 2 h , take the solid, wash three times with DMF and methanol respectively, and dry to obtain a hollow spherical cobalt complex.
[0043] The hollow spherical cobalt complex was calcined in a tube furnace in an air atmosphere. The initial temperature was 20 °C, the heating rate was 5 °C / min, and the cobalt complex was held at 350-400 °C for 3 hours. Cobalt oxide.
[0044] According to hollow spherical cobalt oxide with NaH 2 PO 4 The mass ratios w...
Embodiment 2
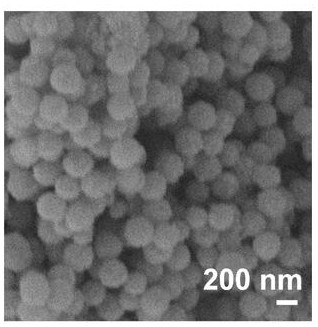
[0046] Put 0.55 g of Co(NO 3 ) 2 . 6H 2 O was dissolved in 15 mL methanol solution to prepare cobalt salt methanol solution, 0.62 g of C4H6N2 ligand was dissolved in 15 mL methanol solution to prepare organic ligand methanol solution, washed with methanol for 3 times after ultrasonication for 30 minutes, and dried to obtain dodecahedral Cobalt complex.
[0047] The dodecahedral cobalt complex was calcined in a tube furnace in an air atmosphere. The initial temperature was 20 °C, the heating rate was 5 °C / min, and the dodecahedron was maintained at 350 to 400 °C for 3 hours. Hedral cobalt oxide.
[0048] Cobalt oxide with NaH according to dodecahedron 2 PO 4 The mass ratio is 1:10 (the phosphating ratio is 1:10) for the phosphating reaction, and the dodecahedral cobalt oxide and NaH are weighed respectively. 2 PO 4 , and NaH 2 PO 4 Put a porcelain boat, the dodecahedral cobalt oxide is put into another porcelain boat, and it is calcined in a tube furnace under nitroge...
Embodiment 3
[0050] Put 0.15 g Co(NO 3 ) 2 . 6H 2 O, 0.13 g Na 2 C 2 O 4 and 0.14 g C 6 H 12 N 4 It was added to a mixed solution of 20 mL of deionized water and 5 mL of ethanol, stirred at room temperature for 4 hours, and the solid was washed with water and ethanol—three times and then dried to obtain a rod-shaped cobalt compound.
[0051] The rod-shaped cobalt compound was calcined in a tube furnace in an air atmosphere, and the rod-shaped cobalt oxide was obtained by calcination at a starting temperature of 20 °C, a heating rate of 5 °C / min, and 350-400 °C for 3 hours. .
[0052] According to rod-shaped cobalt oxide with NaH 2 PO 4 The mass ratio is 1:10 (the phosphating ratio is 1:10) to carry out the phosphating reaction, and the rod-shaped cobalt oxide and NaH are weighed respectively. 2 PO 4 , and NaH 2 PO 4 Put into a porcelain boat, the rod-shaped cobalt oxide is put into another porcelain boat, calcined in a tube furnace under nitrogen atmosphere, nitrogen is conn...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com