Metal complex catalyst and synthesis process of diphenyl carbonate
A technology of metal complexes and catalysts, which is applied in the field of metal complex catalysts and the synthesis process of diphenyl carbonate, can solve the problems of high cost, poor selectivity of diphenyl carbonate catalysts, easy corrosion of chlorine, and achieve low cost , high catalyst activity and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] S1: Measure the saturated water volume of the carrier
[0078] Weigh 98.7 parts of zirconium phosphate, add water drop by drop until saturated, and record the volume V1 of the water added dropwise.
[0079] S2: Preparation of metal complex catalysts
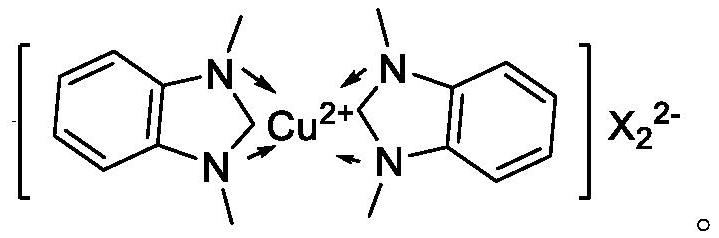
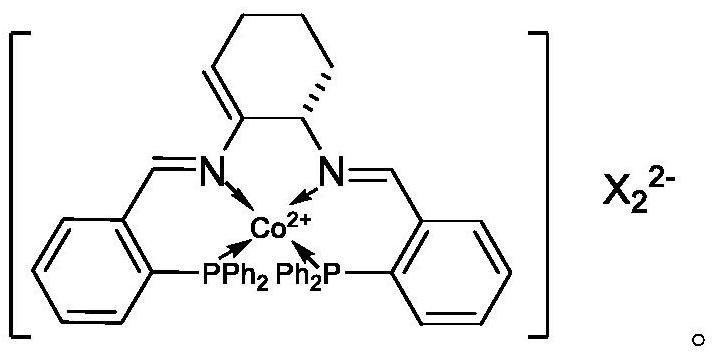
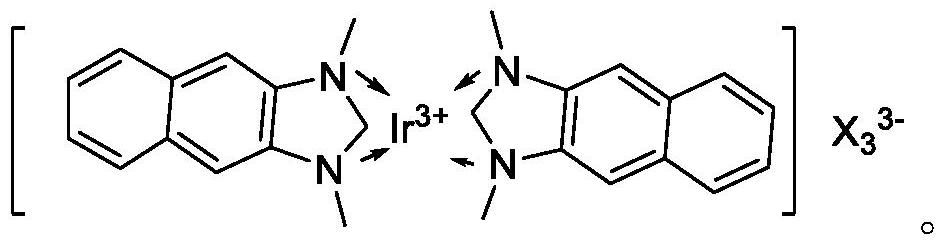
[0080] Weigh 1 part of Cu(NN-DBDMID) 2 I 2 , 0.2 part of Co(PNNP)I 2 and 0.1 part Ir(NN-DBNMID) 2 I 3 Dissolve it in water, adjust the pH to 7.5 with ammonia water, and make the total volume V1 to obtain an impregnation solution. Immerse 98.7 parts of zirconium phosphate in the above impregnating solution until all the impregnating solution is absorbed, then dry at 110-120°C for 4 hours, grind through a 200-mesh sieve, and extrude into strips to obtain the metal complex catalyst, denoted as YDYC- 01.
[0081] YDYC-01 includes 1 part of Cu(NN-DBDMID) 2 I 2 , 0.2 part Co(PNNP)I 2 , 0.1 part of Ir(NN-DBNMID) 2 I 3 and 98.7 parts of zirconium phosphate.
Embodiment 2
[0083] S1: Measure the saturated water volume of the carrier
[0084] Weigh 97.3 parts of zirconium phosphate, add water drop by drop until saturated, record the volume V2 of water added dropwise.
[0085] S2: Preparation of metal complex catalysts
[0086] Weigh 2 parts of Cu(NN-DBDMID) 2 I 2 , 0.5 part of Co(PNNP)I 2 and 0.2 parts Ir(NN-DBNMID) 2 I 3 Dissolve it in water, adjust the pH to 7.5 with ammonia water, and make the total volume V2 to obtain an impregnation solution. Immerse 97.3 parts of zirconium phosphate in the above impregnating solution until all the impregnating solution is absorbed, then dry at 110-120°C for 4 hours, grind through a 200-mesh sieve, and extrude into strips to obtain the metal complex catalyst, denoted as YDYC- 02.
[0087] YDYC-02 includes 2 parts of Cu(NN-DBDMID) 2 I 2 , 0.5 part Co(PNNP)I 2 , 0.2 parts Ir(NN-DBNMID) 2 I 3 and 97.3 parts of zirconium phosphate.
Embodiment 3
[0089] S1: Measure the saturated water volume of the carrier
[0090] Weigh 95.9 parts of β molecular sieve, add water drop by drop until saturated, and record the volume V3 of the water added dropwise.
[0091] S2: Preparation of metal complex catalysts
[0092] Weigh 3 parts of Cu(NN-DBDMID) 2 I 2 , 1 Co(PNNP)I 2 and 0.1 part Ir(NN-DBNMID) 2 I 3 Dissolve it in water, adjust the pH to 7.5 with ammonia water, and make the total volume V3 to obtain an impregnation solution. Immerse 97.3 parts of β molecular sieve in the above impregnation solution until all the impregnation solution is absorbed, then dry at 110-120°C for 4 hours, grind through a 200-mesh sieve, and extrude into strips to obtain the metal complex catalyst, denoted as YDYC- 03.
[0093] YDYC-03 includes 3 parts of Cu(NN-DBDMID) 2 I 2 , 1 part Co(PNNP)I 2 , 0.1 part of Ir(NN-DBNMID) 2 I 3 and 95.9 parts beta molecular sieves.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com