Anti-osteoporosis compound as well as derivatives, pharmaceutical composition, preparation method and application thereof
A technology for anti-osteoporosis compounds, which is applied in the field of anti-osteoporosis compounds and their derivatives, can solve the problems of uncertainty in taking estrogen, and achieve the effects of a wide range of drug targets, easy operation, and simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0130] Preparation of test cells:
[0131] C57BL / 6 mice were killed by neck dislocation, soaked in 75% alcohol for disinfection, and the long bones of the hind limbs (femur and tibia) were peeled off under aseptic conditions, and the attached soft tissues were removed. Rinse out, filter the cell suspension with a cell sieve, seed the cells in a 10 cm diameter culture plate after quantification, and store in 5% CO 2 Cultivate overnight under saturated humidity conditions, centrifuge the next day to take supernatant non-adherent cells, replace with fresh complete medium (containing 30ng / mL M-CSF) and continue to culture for two days to obtain bone marrow osteoclast precursor cells.
[0132] Inhibition rate (PR%)=[enzyme activity (negative control group)-enzyme activity (experimental group)] / [enzyme activity (negative control group)-enzyme activity (blank control group)]×100%.
Embodiment 1
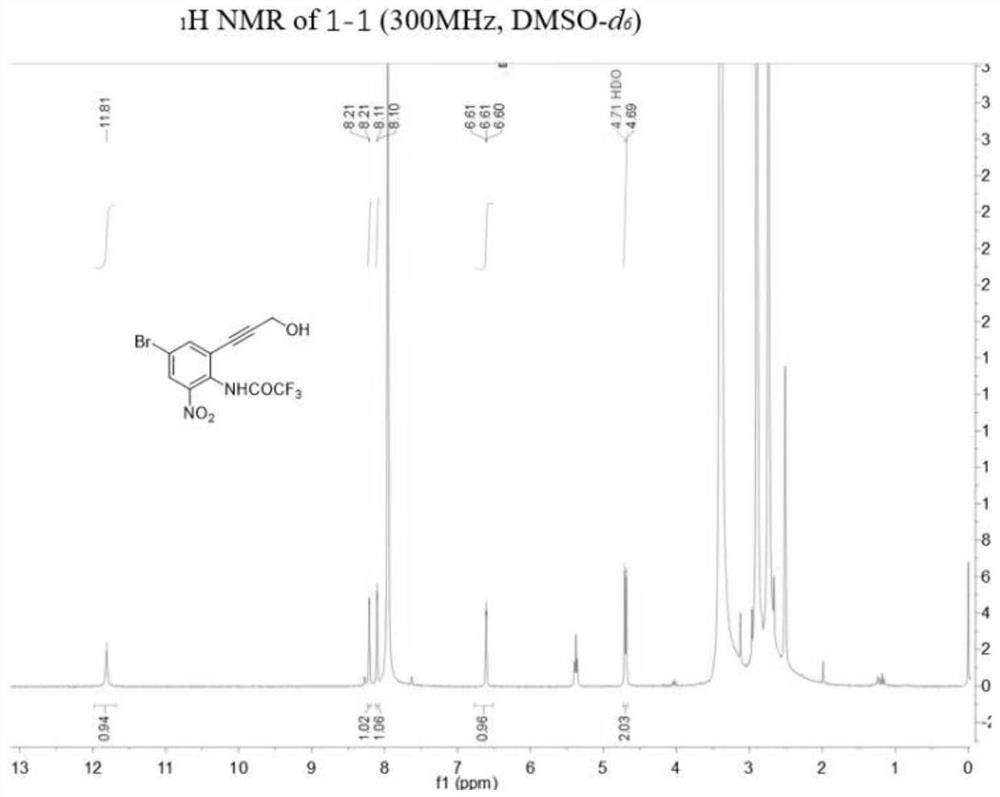
[0133] Example 1: N-(4-bromo-2-(3-hydroxyprop-1-yn-1-yl)-6-nitrophenyl)-2,2,2-trifluoroacetamide (compound 1- 1) Synthesis of
[0134]
[0135] Among them: (a) Ag 2 SO 4 , I 2 ,CH 3 OH, r.t., overnight; (b) (CF 3 CO) 2 O, anhydrous Et 3 N, anhydrous DCM, 0℃, 5min; r.t., 2h; (c) propargyl alcohol, PdCl 2 (PPh 3 ) 2 ,CuI,Et 3 N,DMF,N 2 , r.t., 12h.
[0136] (1) Synthesis of 4-bromo-2-iodo-6-nitroaniline (compound 1a-1)
[0137] Add iodine (9.70g, 38.23mmol) to a 500mL eggplant-shaped bottle, then add 250mL methanol to the bottle to dissolve the iodine, then add silver sulfate (11.92g, 38.23mmol) and 4-bromo-2-nitroaniline in turn (5.00 g, 25.49 mmol), stirred overnight at room temperature. After the reaction was detected by TLC, it was filtered and the reaction solvent was distilled off. After diluting the residue with dichloromethane (100mL), add saturated sodium thiosulfate solution (100mL), then extract with dichloromethane (100mL×3), dry over anhydrous sodi...
Embodiment 2
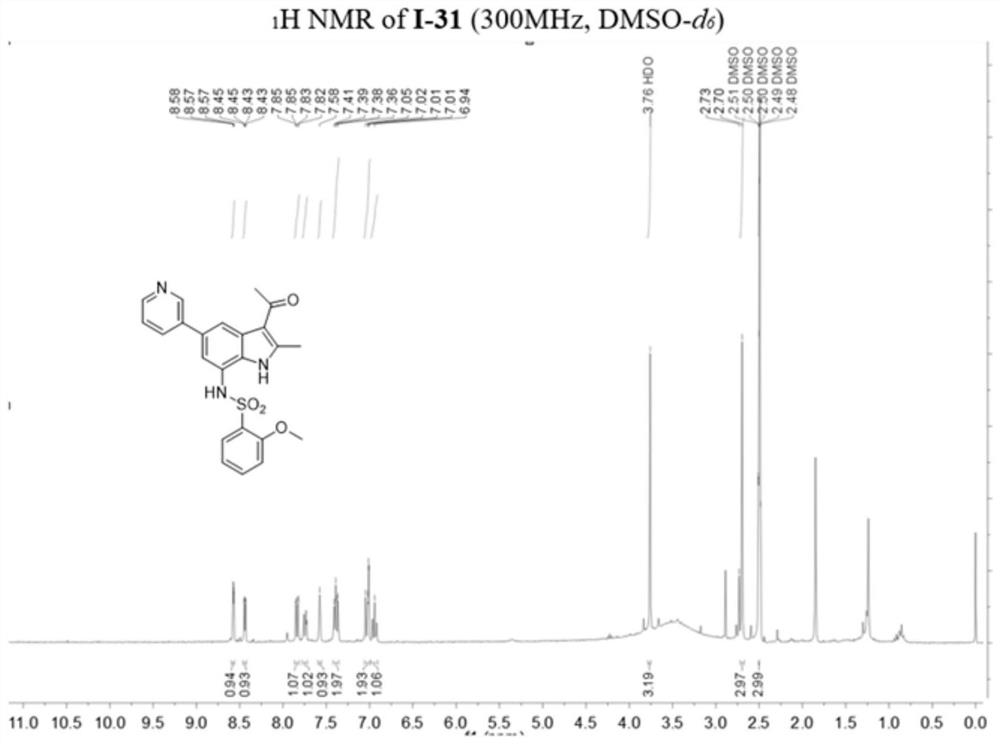
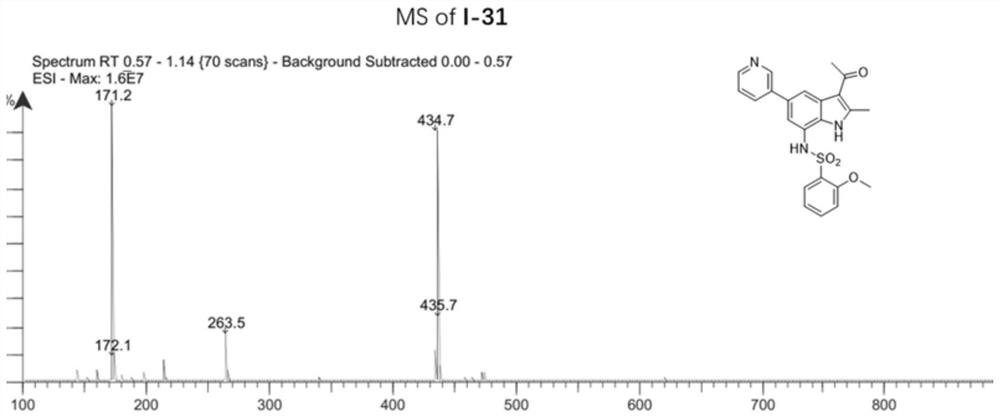
[0142] Example 2: 1-(7-(azetidin-3-ylamino)-2-methyl-5-(pyridin-3-yl)-1H-indol-3-yl)ethane-1 -Synthesis of ketone (compound 1-30)
[0143]
[0144] Among them: (a) 3-pyridylboronic acid, Pd(Ph 3 P) 4 , K 2 CO 3 ,1,4-dioxane,H 2 O, 100℃, 12h; (b) ethyl chloroformate, anhydrous pyridine, 0℃, 5min; r.t., 2h; (c) Pd(PPh 3 ) 4 , anhydrous Et 3 N,HCO 2 H,CH 3 CN,N 2 ,80℃,2h; (d)Pd / C,H 2 ,CH 3 OH / THF (v / v, 1 / 1), r.t., 5h; (e) EtOH, NaBH 4 ,rt,2h; (f)acetyl chloride,Et 2 AlCl,AlCl 3 ,N 2 , anhydrous DCM, 0℃, 5min; r.t., 5h; (g) CF 3 COOH, DCM, r.t., 1h.
[0145](1) 2,2,2-trifluoro-N-(2-(3-hydroxyprop-1-yn-1-yl)-6-nitro-4-(pyridin-3-yl)phenyl)ethyl Synthesis of Amide (Compound 2-1)
[0146] Compound 1-1 (100mg, 272.42μmol), 3-pyridineboronic acid (49.8mg, 408.63μmol), tetrakis (triphenylphosphine) palladium (31.5mg, 27.24μmol) and potassium carbonate (113.0mg, 817.26μmol) were added To a 25 mL three-necked round bottom flask, after replacing the air in the reacti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com