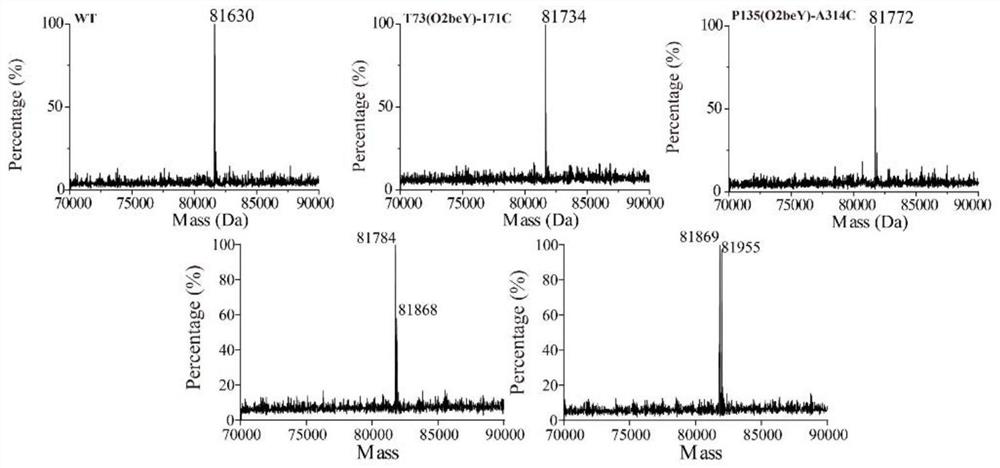
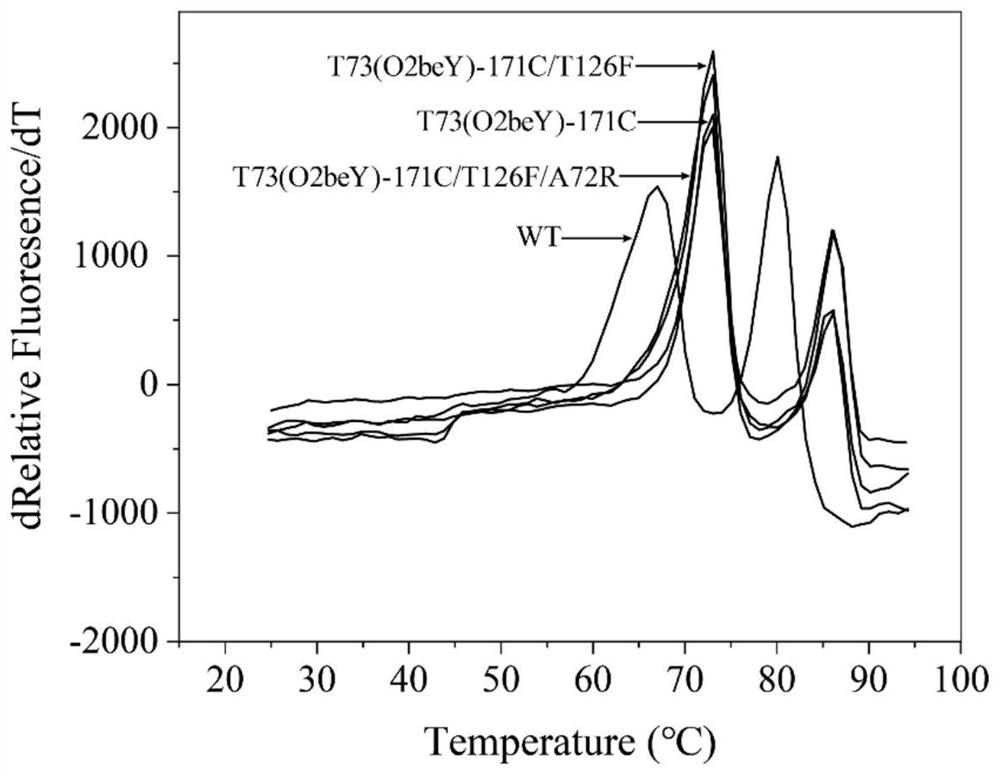
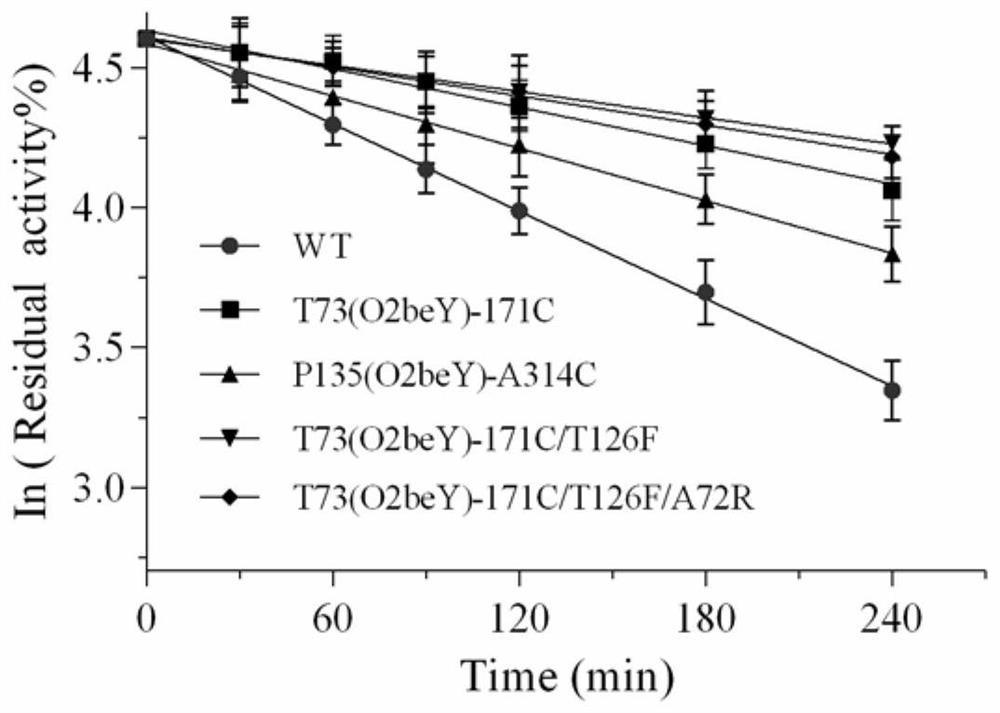
Heat-resistant neutral pullulanase mutant and application thereof
A pullulanase and mutant technology, which is applied in the field of mutants of thermostable neutral pullulanase, can solve the problems of poor activity and stability of wild-type pullulanase, and achieves improved thermostability and secretion. The effect of improved efficiency and improved thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1. Construction of recombinant plasmids
[0038] (1) Codon-optimizing the pullulanase gene from Bacillusthermoleovoran shown in SEQ ID NO.2 according to the codon preference rule of Escherichia coli, and the optimized nucleotide sequence is shown in SEQ ID NO.3;
[0039] (2) Use NcoI endonuclease and XhoI endonuclease to linearize the expression vector pET28a, and utilize NcoI endonuclease and XhoI endonuclease to double pullulanase gene (Btpul) shown in SEQ ID NO.3 Enzyme digestion treatment; the product after enzyme digestion treatment passed T 4 DNA ligase was connected to obtain wild-type pullulanase recombinant plasmid; the recombinant plasmid was transferred into E.coliJM109 competent cells, and the plasmid was extracted from positive transformants (plasmid extraction kit was purchased from OMEGA Company) to obtain wild-type Btpul recombinant plasmids.
[0040] (3 using PCR enzyme (purchased from TaKaRa company) adopts two-step PCR or whole plasmid PC...
Embodiment 2
[0103] Embodiment 2. Construction of recombinant bacteria
[0104] 1. Wild-type pullulanase expression strain: preparation of competent cells: referring to the standard operation on the Competent CellPreparation kit (purchased from TaKaRa Company), the preserved Escherichia coli was streaked and cultured to an LB-free plate, and picked Take the grown single colony to an LB test tube without antibiotics, and culture overnight at 37°C and 200rpm for 8-12 hours; after that, transfer to a 250mL Erlenmeyer flask containing 20% LB antibiotic-free medium at an inoculum size of 2% , and the culture conditions were as described above at 37°C and 200 rpm for 8-12 hours overnight; 600 After about 0.6-0.8, put the 250mL Erlenmeyer flask on ice for 30min; divide the bacterial solution into sterilized 50mL centrifuge tubes in 25mL aliquots; centrifuge at 8000rpm for 5min at 4°C and discard the supernatant , add 10% solution A to gently resuspend the bacteria; centrifuge again at 4°C, 600...
Embodiment 3
[0109] Embodiment 3. Expression and purification of pullulanase
[0110] (1) Expression of wild-type pullulanase
[0111] The recombinant bacteria containing wild-type Btpul were inoculated into 5 mL containing kanamycin (50 μg·mL -1 ) in LB test tubes at 37°C and 200rpm for 8-12h; transfer the culture solution to 20% autoinduction medium (10g·L -1 Tryptone, 5.0g·L -1 Yeast extract, 10g·L -1 α-lactose, 5.0g·L -1 Glycerin, 1.0g·L -1 Glucose, 7.1g·L -1 Na 2 HPO 4 , 6.8g·L -1 K H 2 PO 4 , 2.67g L -1 NH 4 Cl, 0.71g L -1 Na 2 SO 4 and 0.25g·L -1 MgSO 4 ; pH7.5) in a 250mL Erlenmeyer flask, and add kanamycin to its final concentration of 50μg·mL -1 , cultivated at 37°C and 200rpm for 2-3h; lowered the temperature to 17°C, and continued to cultivate for 48-60h to express the wild-type recombinant protein.
[0112] (2) Expression of pullulanase mutants
[0113] The double-plasmid recombinant bacteria containing the recombinant plasmid of pullulanase mutant gene and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com