Diphenylamino-truxene-BODIPY derivative ternary system organic dye as well as preparation method and application thereof
A diphenylamine-based, ternary system technology, applied in the direction of organic dyes, styryl dyes, methine-based/polymethine-based dyes, etc., to achieve mild and efficient reaction conditions, enhanced light absorption capacity, and good application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
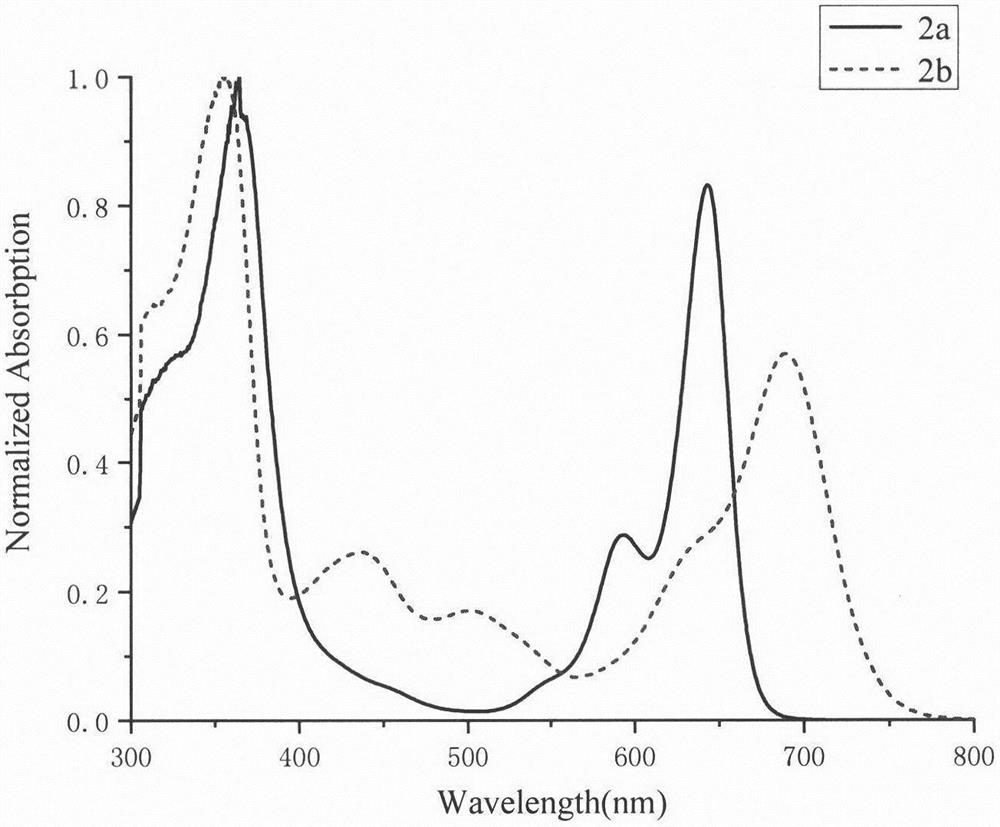
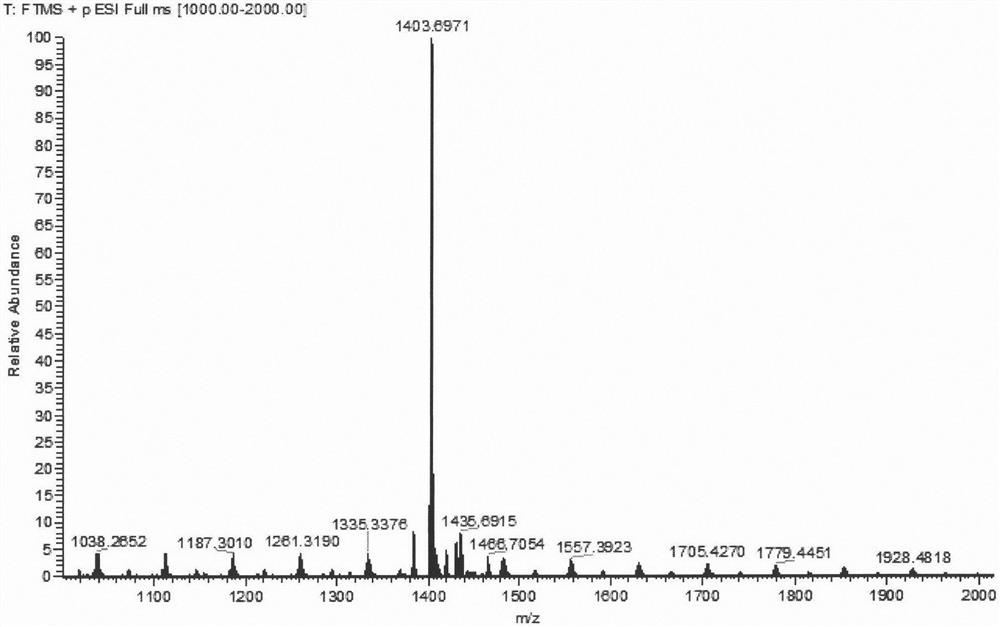
[0026] Compound 2-bromo-7,12-bis(N,N-diphenylamino)-tripolyindene (92.4 mg, 0.1 mmol), compound 1,7-dimethyl-3,5-bis (4-Methoxystyryl)-8-(boronate)-BODIPY 1a (103 mg, 0.15 mmol), anhydrous cesium carbonate (330 mg, 1 mmol) were dissolved in 20 mL of tetrahydrofuran, and 2 mL of water was added thereto with 2mL of methanol, tetrakis(triphenylphosphine)palladium (18mg, 0.015mmol) was quickly added thereto under the protection of argon, and the temperature was raised to 65°C for 12h. After the reaction was completed, the solvent was spin-dried, extracted with dichloromethane, washed with saturated sodium chloride solution, the organic layer was dried over anhydrous sodium sulfate, distilled under reduced pressure to spin-dry the solvent, and dichloromethane-petroleum ether (v:v=1: 1) was separated and purified by silica gel chromatography as the eluent to obtain dark brown solid 2a (66 mg). 1 H NMR (CDCl 3 , 600MHz, ppm): δ8.39(d, J=10.2Hz, 1H), 8.19(d, J=3.6Hz, 1H), 8.12(d, J=...
Embodiment 2
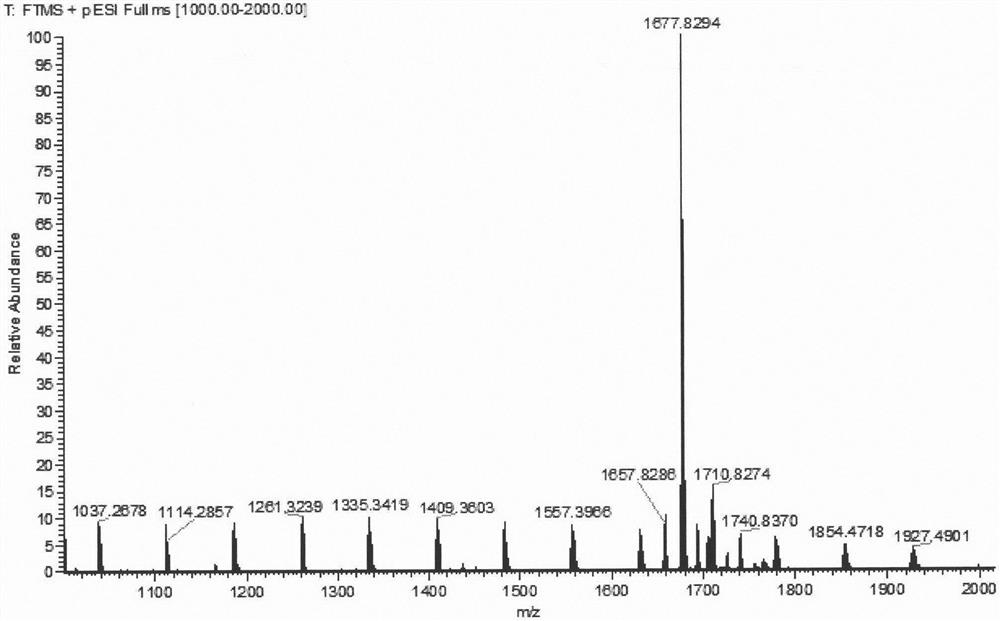
[0029] Compound 2-bromo-7,12-bis(N,N-diphenylamino)-tripolyindene (92.4 mg, 0.1 mmol), compound 1,7-dimethyl-3,5-bis (4-N,N-Diphenylaminostyryl)-8-(boronate)-BODIPY 1b (144.1 mg, 0.15 mmol), anhydrous cesium carbonate (330 mg, 1 mmol) were dissolved in 20 mL of tetrahydrofuran, and 2 mL of water and 2 mL of methanol were added thereto, and tetrakis(triphenylphosphine)palladium (18 mg, 0.015 mmol) was quickly added thereto under the protection of argon, and the temperature was raised to 68° C. for 12 h. After the reaction was completed, the solvent was spin-dried, extracted with dichloromethane, washed with saturated sodium chloride solution, the organic layer was dried over anhydrous sodium sulfate, distilled under reduced pressure to spin-dry the solvent, and dichloromethane-petroleum ether (v:v=1: 1) was separated and purified by silica gel chromatography as the eluent to obtain dark brown solid 2b (40mg). 1 H NMR (CDCl 3 , 600MHz, ppm): δ8.38(d, J=8.4Hz, 1H), 8.19(d, J=8....
Embodiment 3
[0032] In the present invention, 2a and 2b in the embodiment 1 and 2 are used as electron donors and PC71BM and Y6 as electron acceptors to prepare solar cells respectively, and the efficiency of the device is tested respectively. The photoelectric conversion efficiency current density-voltage curve is shown in Figure 4 . The performance of the above devices is summarized in the following table:
[0033]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com