Recombinant protein and porcine epidemic diarrhea vaccine composition
A porcine epidemic diarrhea and vaccine composition technology, applied in the biological field, can solve the problems of large inoculation, insufficient immunity, and inapplicability of emergency vaccination, and achieve low production cost, convenient large-scale production, and good immune response Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Construction of recombinant plasmids for eukaryotic expression of recombinant proteins
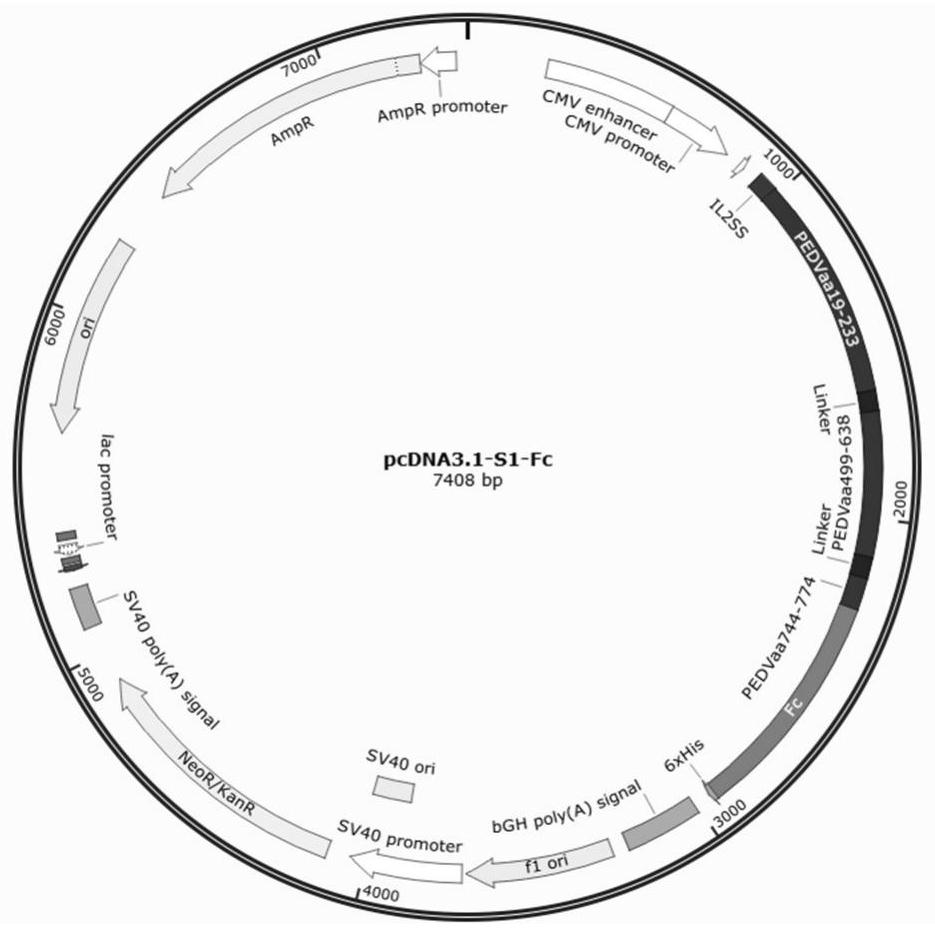
[0034] In this example, based on the S gene sequence of PEDV AH2012 / 12 strain (GenBank: KU646831.1), a fusion gene with the DNA sequence shown in SEQ ID NO.6 was synthesized by Nanjing GenScript Biotechnology Co., Ltd., denoted as S1- Fc. The fusion gene S1-Fc contains three truncated fragments of the S protein gene, which are the N-terminal domain (NTD, aa19-233) with sialic acid binding activity in the S1 subunit, the neutralizing antigen core region (COE, aa499-638) and the B cell recognition epitope (aa744-774) in the S2 subunit, these three truncated fragments are sequentially connected through a linker polypeptide (linker), and then fused with the Fc fragment of the pig IgG antibody.
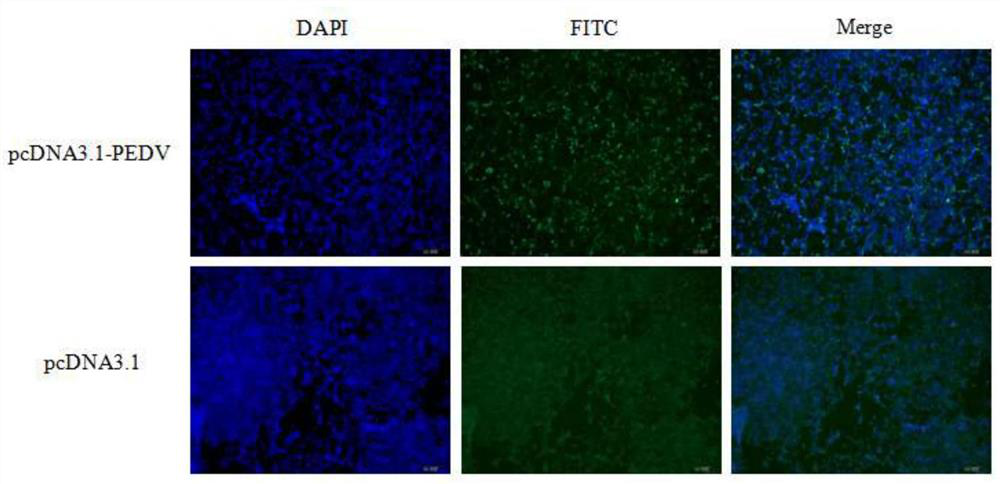
[0035] The synthetic fusion gene was cloned into the plasmid vector pcDNA3.1 by conventional molecular biological means, and the recombinant plasmid pcDNA3.1-S1-Fc for expressing t...
Embodiment 2
[0040] Embodiment 2: prepare the recombinant protein of eukaryotic expression
[0041] In this example, Expi293 TM expression systems to produce large quantities of recombinant proteins. The used ExpiFectamine of this embodiment TM 293 transfection kit was purchased from Gibco.
[0042] The specific operation is as follows:
[0043] (1) in 30mL Expi293 TM Inoculate 6×10 in the expression medium 7 Expi293F TM Living cells;
[0044] (2) At 37°C, 8% CO 2 , Incubating the cells in an orbital shaker at 125rpm;
[0045] (3) Use an automatic cell counter to determine cell number and viability, and the cell density should be 3×10 6 ~5×10 6 cells / mL, the cell viability should be >95%;
[0046] (4) Use 25.5mL Expi293 in a 125mL flask TM Expression medium will be 7.5 x 10 7 Cells were diluted to 2.9×10 6 a / mL;
[0047] (5) Dilute 30 μg of pcDNA3.1-S1-Fc eukaryotic expression plasmid with Opti-MEM in tube A to a total volume of 1.5 mL, and dilute 81 μL of ExpiFectamine wi...
Embodiment 3
[0064] Embodiment 3: preparation vaccine composition
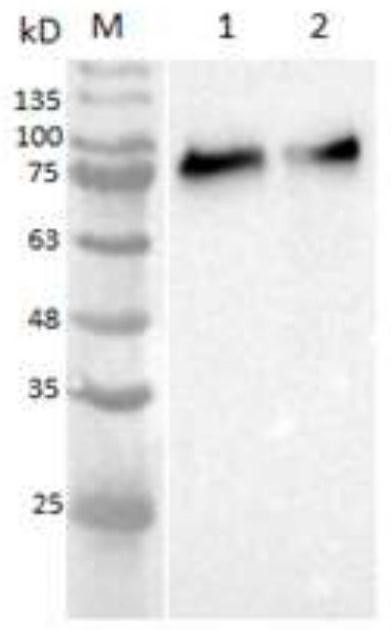
[0065] The commercially available Freund's adjuvant and the layered double metal hydroxide (LDH) adjuvant prepared by the inventors were mixed with the purified recombinant protein prepared in Example 2 to prepare vaccine compositions.
[0066] The layered double hydroxide (LDH) adjuvant was prepared as follows: First, prepare 15 mL of mixed salt solution containing 8.0 mmol of Mg(NO 3 ) 2 and 4.0mmol of Al(NO 3 ) 3 ; Next, prepare 20mL of 4.0mol / L NaOH solution, add 20mmol of lactic acid (88%), stir vigorously for 2h to obtain NaOH mixture; then, add 15mL of mixed salt solution to 11mL of NaOH mixture for reaction, and react The resulting precipitate was sonicated in an ice bath for 10 minutes, centrifuged at 5000 rpm for 10 minutes to obtain a pure LDH slurry, and the LDH slurry was washed twice with water, and then manually dispersed in 20 mL of water to obtain the prepared LDH adjuvant. Use Nicomp 380Z3000 nano p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com