Application of arabidopsis thaliana UGT74F2 in catalyzing phenyllactic acid to synthesize phenyllactoyl glucose
A technology for catalyzing phenyllactic acid and Arabidopsis, applied in biochemical equipment and methods, transferases, enzymes, etc., can solve the problems of limited accumulation and weak catalytic activity, and achieve the effect of increasing alkaloid content and expanding application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
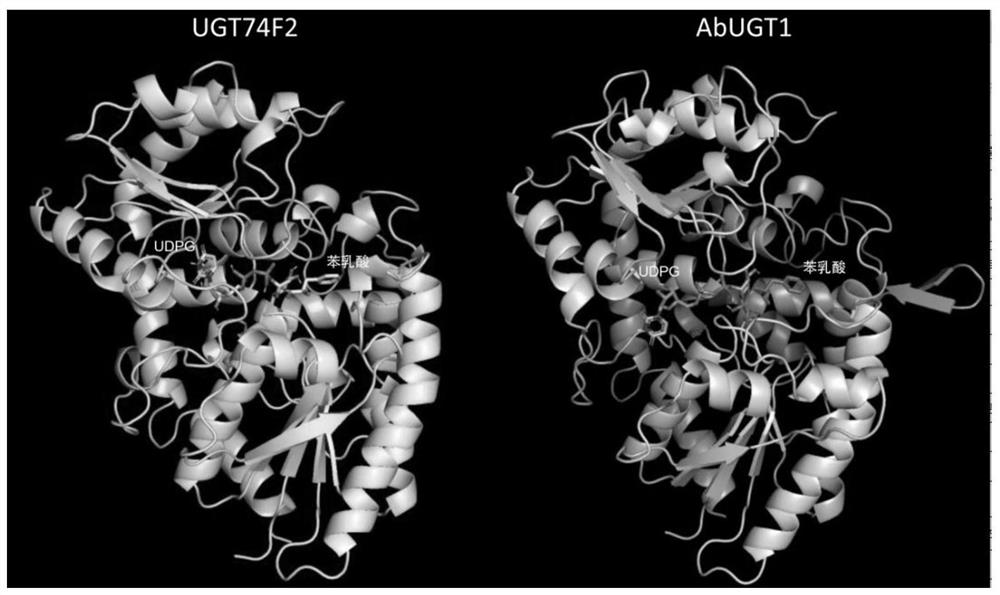
[0023] Example 1. Molecular docking screening for potential glucosylphenyllactate transferases
[0024] 909 glucosyltransferase protein crystal structures were downloaded from the Protein Data Bank PDB. Homology modeling of belladonna AbUGT1 was performed using Swissmodel to obtain its protein structure model. Download the three-dimensional structures of D-(+)-Phenyllactic acid (PLA) and Uridine Diphosphate Glucose (UDPG) from the PubChem database. Using the Autodock Vina software, the glycosyltransferase and AbUGT1 protein models from the PDB database were used to dock PLA and UDPG respectively. Each docking calculation was repeated 10 times, and the binding free energy was calculated. Finally, pymol was used to generate the complex model of glycosyltransferase, PLA and UDPG, and the substrate binding pocket was analyzed.
[0025] Molecular docking calculation results show that the binding free energy of AbUGT1 of belladonna to PLA is -5.5kcal.mol -1 , the binding free ene...
Embodiment 2
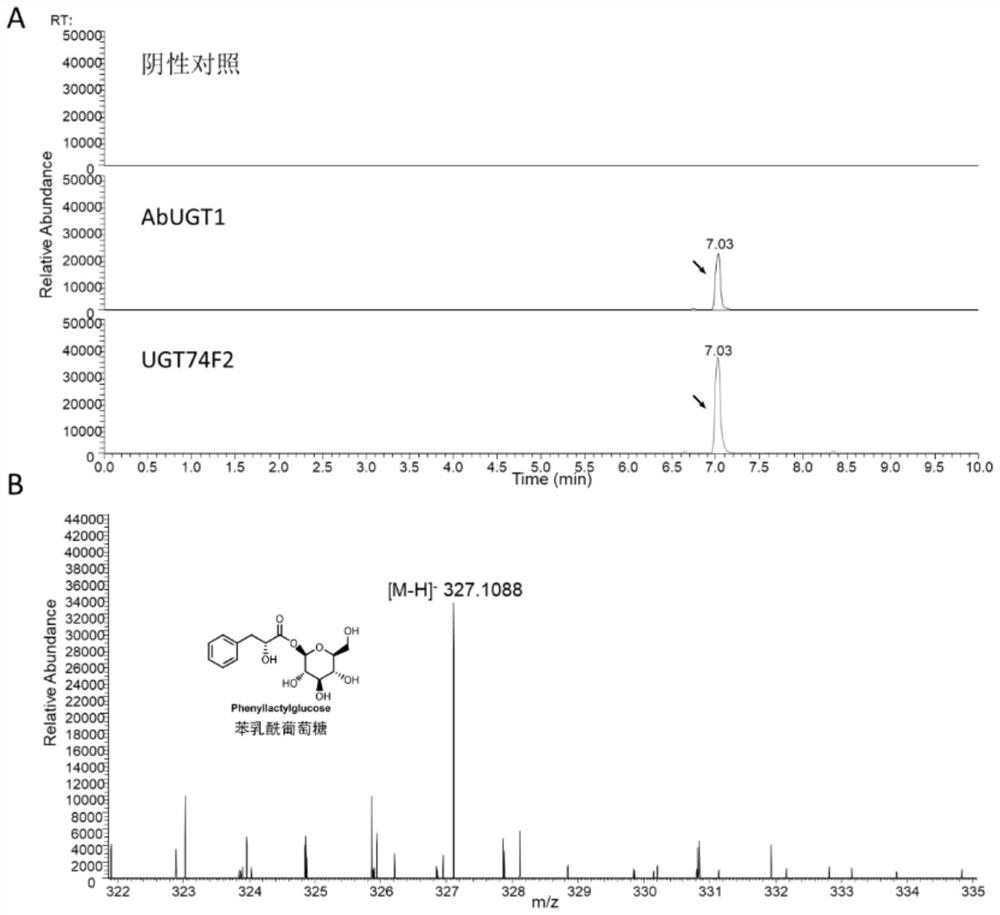
[0026] Embodiment 2, enzyme activity analysis
[0027] (1) Arabidopsis total RNA extraction
[0028] Take an appropriate amount of Arabidopsis plant tissue, put it in liquid nitrogen for quick freezing, grind it into a fine powder at low temperature, add it to a 1.5mL Eppendorf (EP) centrifuge tube containing 1mL of lysate, shake and mix well, and follow the TIANGEN kit Total RNA was extracted according to the instruction manual. The integrity of the total RNA was detected by agarose gel electrophoresis, and the concentration of the extracted RNA was measured and analyzed on an ultra-micro ultraviolet spectrophotometer.
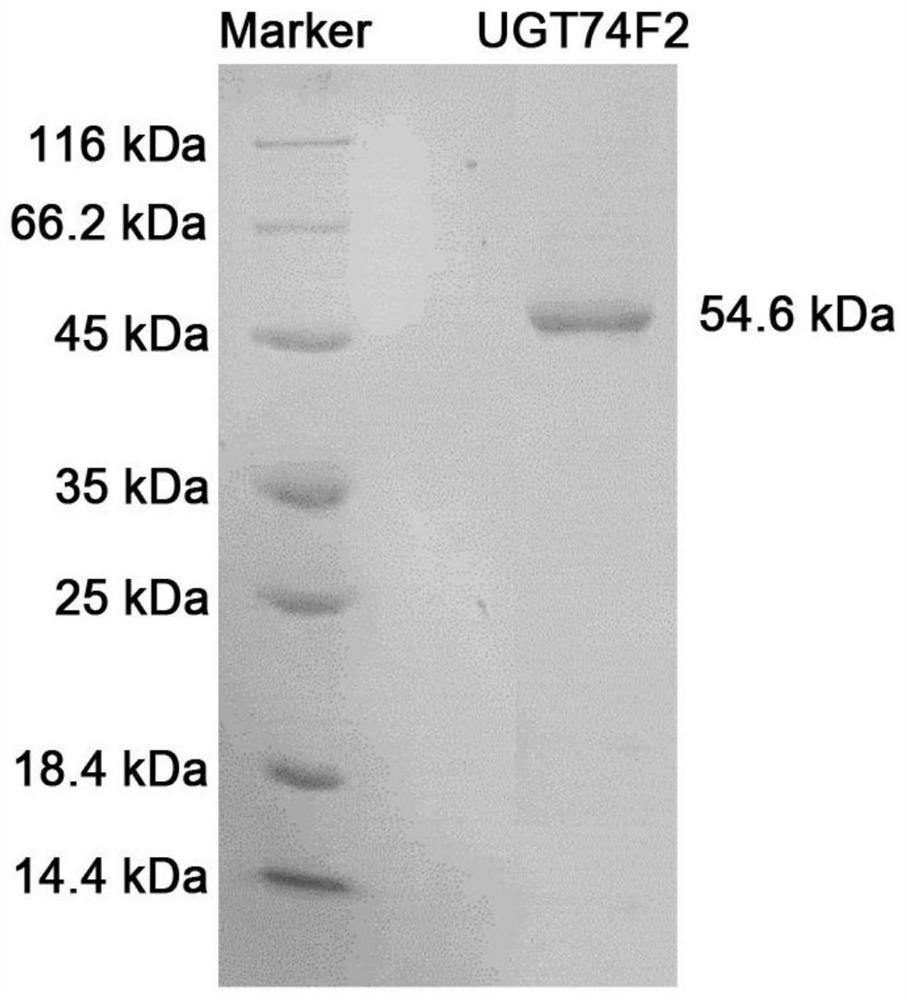
[0029] (2) Gene cloning and prokaryotic expression vector construction
[0030] Using the extracted total RNA as a template, cDNA was synthesized according to the instructions of the Tiangen FastKing cDNA First Strand Synthesis Kit; the UGT74F2 sequence was obtained from the NCBI public database, and the recombinant primers were designed in combination with...
Embodiment 3
[0048] Embodiment 3, the effect of UGT74F2 in tropane alkaloid plant metabolism engineering
[0049] (1) Construction of plant overexpression vector
[0050] Enzyme activity experiments in vitro have shown that UGT74F2 has higher catalytic efficiency than AbUGT1, suggesting that UGT74F2 may have a better role in promoting the biosynthesis of conchine downstream. Therefore, in order to understand the promotion effect of overexpression of UGT74F2 and AbUGT1 on the synthesis of downstream alkaloids in vivo, this study constructed overexpression vectors and compared plant metabolic engineering with belladonna hairy roots to evaluate the role of the above two genes in tropicane biogenesis. Significance in base biosynthesis.
[0051] Based on the UGT74F2 coding region sequence obtained above, recombinant primers were designed in conjunction with the overexpression vector pBI121. Primers were designed as follows:
[0052] 121-UGT74F2-F: 5'-cgggggactctagaggatccatggagcataagagaggaca-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| binding free energy | aaaaa | aaaaa |
| binding free energy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com