Biological agent for preventing and relieving joint sub-health and preparation method thereof
A biological preparation and health technology, applied in the direction of medical preparations containing active ingredients, medical raw materials derived from ferns/filamentous plants, anti-inflammatory agents, etc., can solve low back pain without good curative effect, arthritis is not good Many, single indications and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] The total amount of raw materials is 100 kg. in,
[0068] Phase A materials: 9.0 kg of glycerin, 6.0 kg of propylene glycol, and 2.0 kg of butanediol;
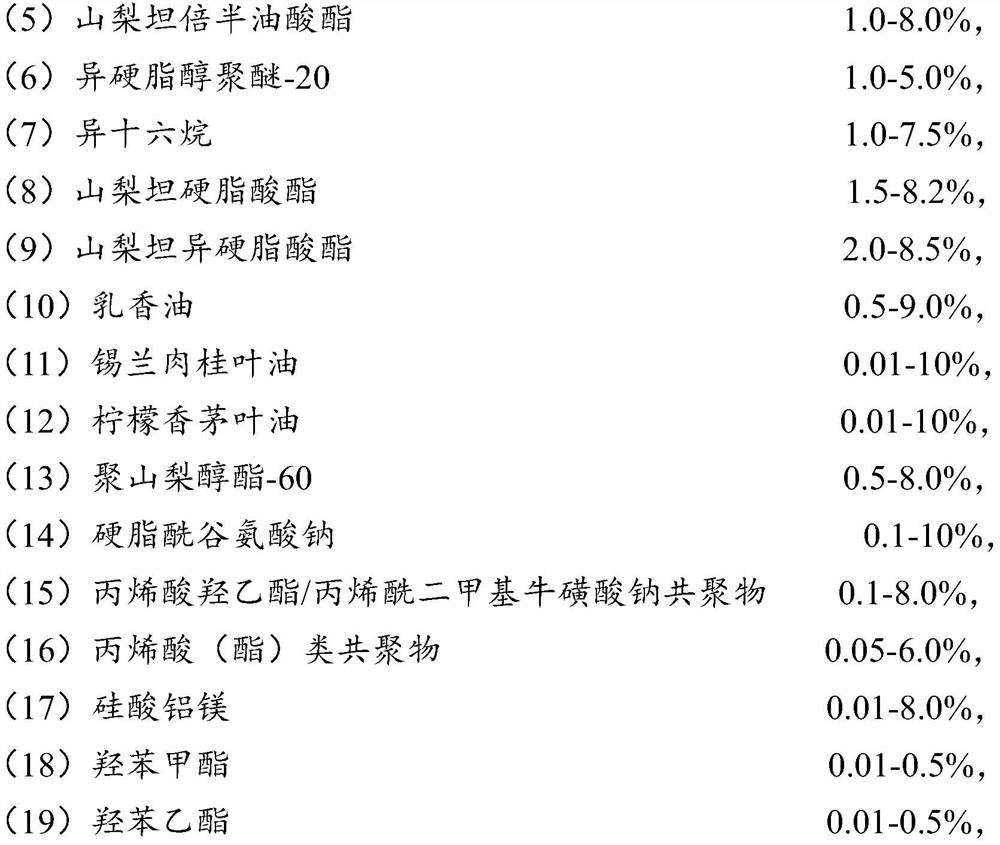
[0069] Phase B materials: 5 kg of sorbitan sesquioleate, 1.2 kg of isosteareth-20, 2.0 kg of isohexadecane, 1.6 kg of sorbitan stearate, 2.3 kg of sorbitan isostearate , Frankincense Oil 0.7 kg, Ceylon Cinnamon Leaf Oil 0.05 kg, Lemongrass Leaf Oil 0.02 kg, Polysorbate-60, 0.8 kg, Sodium Stearoyl Glutamate 0.4 kg, Hydroxyethyl Acrylate / Acryloyl Di 0.3 kg of sodium methyl taurate copolymer, 1.1 kg of acrylic acid (ester) copolymer, 0.05 kg of aluminum magnesium silicate, 0.1 kg of methyl paraben, and 0.02 kg of ethyl paraben;
[0070] Phase C material: 2.0 kg of butanediol, 0.05 kg of tetrahydropiperine;
[0071] Phase D materials: 3.1 kg of Herba chinensis extract, 6.2 kg of Passepartout extract, 4.0 kg of ginger root extract, 5.3 kg of Morinda officinalis fruit extract, 0.15 kg of camphor, 2.5 catties of methyl sali...
Embodiment 2
[0083] The formula of embodiment 2 is identical with embodiment 1, and the difference between embodiment 2 and embodiment 1 is only preparation technology, and the preparation technology of embodiment 2 is as follows:
[0084] (1) Weigh each phase raw material by weight percentage;
[0085] (2) Mix the materials of phase A, add them to the water phase pot, heat to 80°C, and sterilize at a constant temperature for 25 minutes to obtain the water phase materials;
[0086] (3) Mix the materials of phase B, add them into the emulsification pot, heat to 75°C, and sterilize at a constant temperature for 20 minutes to obtain the oil phase materials;
[0087] (4) Under vacuum stirring state, pump 1 / 2 volume of the water phase material obtained in step (2) into the emulsification pot and mix with the oil phase material obtained in step (3), and homogenize at 70°C for 3 minutes, and then sterilize at a constant temperature 25 minutes;
[0088] (5) Heat and dissolve the C-phase material...
Embodiment 3
[0092] The total amount of raw materials is 100 kg. in,
[0093] Phase A materials: 1.0 kg of glycerin, 9.8 kg of propylene glycol, and 4.5 kg of butanediol;
[0094] Phase B material: Sorbitan sesquioleate 3.5 kg, Isosteareth-20 3.0 kg, Isohexadecane 2.3 kg, Sorbitan stearate 1.5 kg, Sorbitan isostearate 2.0 kg , Frankincense Oil 3.2 kg, Ceylon Cinnamon Leaf Oil 0.6 kg, Lemongrass Leaf Oil 0.5 kg, Polysorbate-60 1.0 kg, Sodium Stearoyl Glutamate 0.8 kg, Hydroxyethyl Acrylate / Acryloyl Dimethicone 0.1 kg of sodium taurate copolymer, 0.05 kg of acrylic acid (ester) copolymer, 0.01 kg of aluminum magnesium silicate, 0.01 kg of methyl paraben, and 0.01 kg of ethyl paraben;
[0095] Phase C material: 3.0 kg of butanediol, 4.0 kg of tetrahydropiperine;
[0096] Phase D materials: 2.0 kg of Herba chinensis extract, 4.2 kg of Passepartout extract, 5.0 kg of ginger root extract, 3.0 kg of Morinda officinalis fruit extract, 0.01 kg of camphor, 0.1 catties of methyl salicylate, ascorb...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com