Method for detecting content of gamma-aminobutyric acid in cosmetics
A technology of aminobutyric acid and detection method, applied in the field of gamma-aminobutyric acid detection, can solve the problems that affect the detection accuracy of gamma-aminobutyric acid, it is difficult to detect low-level samples, and the derivative reaction is susceptible to external interference, etc. , to avoid diffusion and overlap, improve detection sensitivity and accuracy, and achieve high accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] Preparation of reference substance: use hydrochloric acid to dissolve the γ-aminobutyric acid reference substance, and filter to obtain the reference substance solution of γ-aminobutyric acid;
[0042] Preparation of the test product: use hydrochloric acid to dissolve the cosmetic test product, and filter to obtain the test product solution;
[0043] Preparation of blank solution: after filtration of hydrochloric acid, a blank solution was obtained;
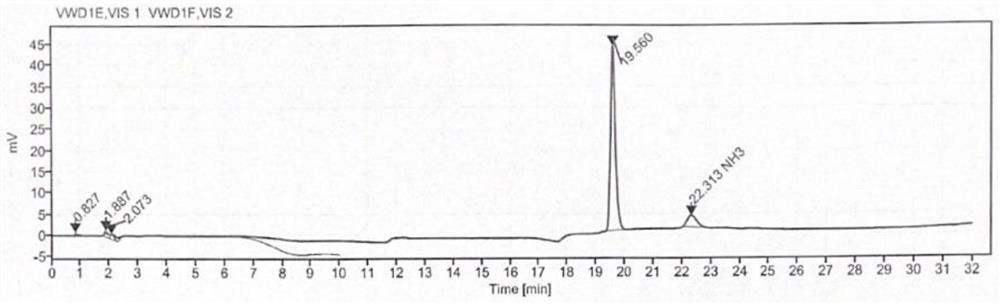
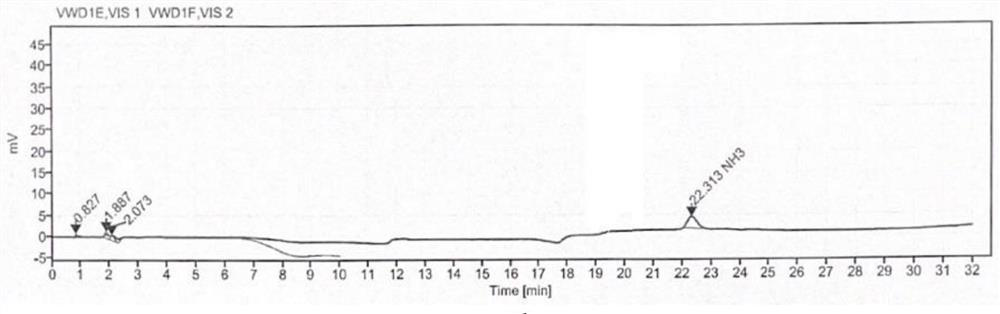
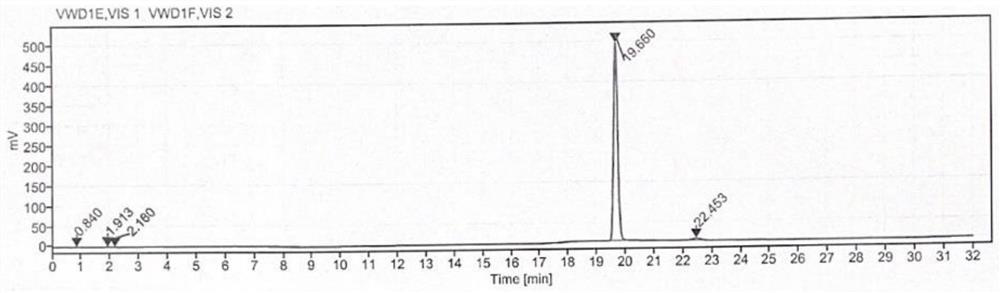
[0044] Determination: use a separation column and a post-column derivatization reaction column to detect the reference solution, the test solution and the blank solution to obtain the content of γ-aminobutyric acid in the cosmetic test product.
[0045] In one embodiment, the concentration of the hydrochloric acid is 0.02mol / L-0.06mol / L, preferably 0.02mol / L.
[0046]For example, the concentration of the hydrochloric acid may be 0.02 mol / L, 0.03 mol / L, 0.04 mol / L, 0.05 mol / L, 0.06 mol / L, and the like.
[0047] The hydroc...
Embodiment 1-1
[0109] 1. Instrument reagents
[0110] LA8080 Amino Acid Analyzer Analytical Balance: METTLER TOLEDO AL104
[0111] Ultrapure water, hydrochloric acid (analytical grade) γ-aminobutyric acid reference substance (99.9%)
[0112] 2. Chromatographic conditions
[0113] Separation column: packed cation exchange resin column (4.4mm×60mm, 5μm model: 852-8534)
[0114] Reaction column: filled with quartz sand (4.6mm×40mm, model: 8L03600)
[0115] Separation flow rate: 0.45ml / min
[0116] Reaction flow rate: 0.3ml / min
[0117] Separation column temperature: 55℃;
[0118] Reaction column temperature: 35°C;
[0119] Injection volume: 20μL;
[0120] Detection wavelength: 336nm
[0121] Gradient elution procedure:
[0122] time / min A% B% C% D% E% R1% R2% R3% 0.0 100 -- -- -- -- 50 50 -- 3.5 -- 100 -- -- -- 50 50 -- 3.6 -- -- 100 -- -- 50 50 -- 6.5 -- -- 100 -- -- 50 50 -- 6.6 -- -- 23 77 -- 50 50 -- ...
Embodiment 1-2
[0145] The sample to be tested in Example 1 was replaced with batch number: 210309, and other conditions were the same as those in Example 1. The content of γ-aminobutyric acid in cosmetics was determined. The results are shown in Table 2, and the detection limit was 3 × 10 -4 ppm.
[0146] The detection result of table 2 embodiment 1-2
[0147]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com