Culture type virus preserving fluid capable of being sterilized by irradiation
A technology for irradiating sterilization and preserving solutions, which is applied in the field of biomedicine and can solve the problems of phenol red losing color, losing real-time detection of pH, and bacterial contamination.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Preparation of preservation solution: 0.85% sodium chloride, 0.001% magnesium sulfate, 0.04% potassium chloride, 0.01% magnesium chloride, 0.006% disodium hydrogen phosphate, 0.006% potassium dihydrogen phosphate, 4-hydroxyethylpiperazineethanesulfonic acid 0.15%, arginine 0.001%-0.05%, cysteine 0.008%, glutamine 0.05%, glycine 0.007%, histidine 0.01%, isoleucine 0.02%, leucine 0.02%, methyl sulfide amino acid 0.003%, phenylalanine 0.006%, serine 0.005%, threonine 0.01%, tryptophan 0.002%, tyrosine 0.01%, valine 0.01%, glucose 1%, sucrose 1%, vitamin C0 .5%, vitamin E 0.1%, amphotericin B 0.01%, gentamicin 0.02%, streptomycin 0.02%, penicillin 0.01% and phenol red 0.004%. The solution was sterilized twice through a 0.1 μm filter.
Embodiment 2
[0017] Comparison of the appearance of the preservation solution before and after 20kGy irradiation sterilization ( figure 1 ).
Embodiment 3
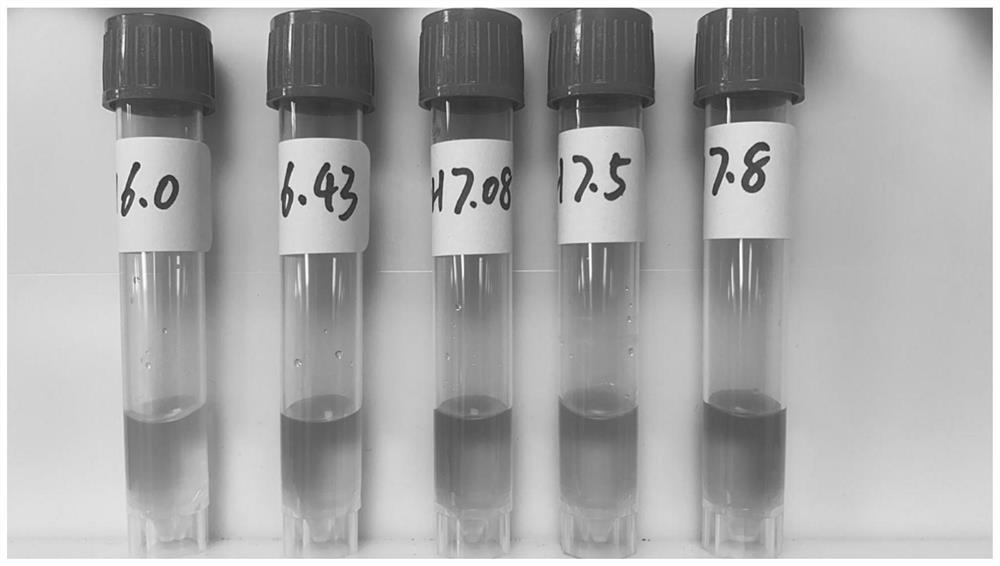
[0019] After 20kGy irradiation sterilization, the phenol red in the preservation solution monitors the change of pH ( figure 2 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com