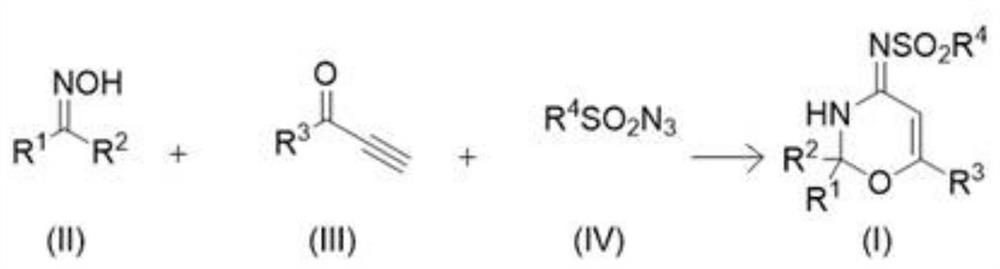
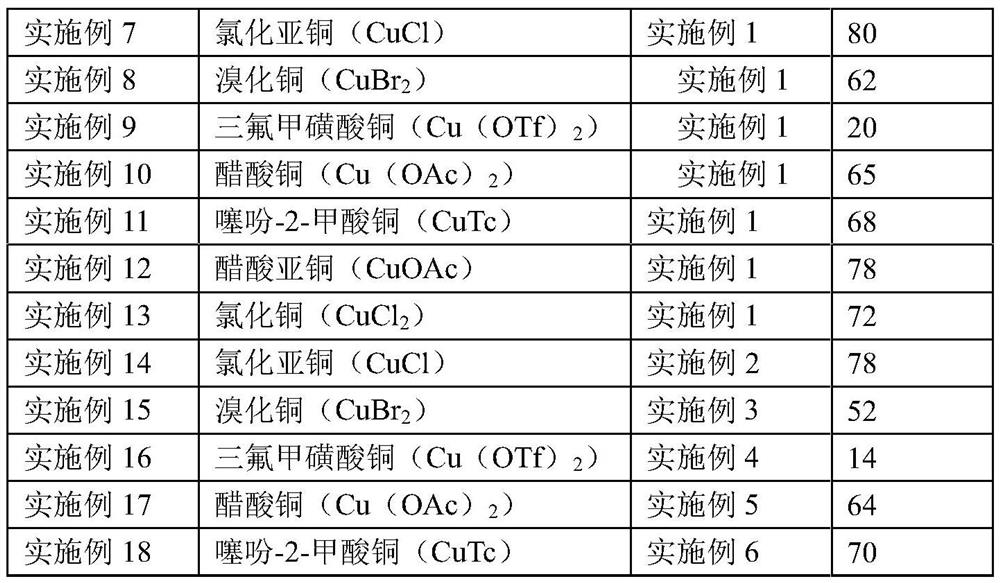
A kind of iminoxazine derivative and preparation method thereof
A technology of iminoxazine and derivatives, applied in the field of organic chemical synthesis, can solve the problems of harsh reaction conditions, many reaction steps, expensive principles, etc., and achieve the effects of high purity, high yield, and high atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Add the compounds of the above formulas (II), (III) and (IV) and cuprous iodide (CuI) into acetonitrile, then raise the temperature to 80° C., and stir and seal the reaction at this temperature for 6 hours.
[0032] Wherein, the molar ratio of formula (II) compound and cuprous iodide (CuI) is 1:0.1; The molar ratio of formula (II) compound and (III), (IV) compound is 1:2:2; The ratio of the compound of formula (II) in moles (mmol) to acetonitrile in milliliters (ml) is 1:3.
[0033] After the reaction, the reaction system was naturally cooled to room temperature, and the solvent was concentrated by rotary evaporation to obtain a crude product. The crude product was subjected to 300-400 mesh silica gel column chromatography, using a mixture of ethyl acetate and petroleum ether as an eluent, wherein acetic acid Ethyl ester and sherwood oil volume ratio 1:4, thus obtain the target product formula (I) compound (C 19 h 20 N 2 o 3 S) with a yield of 82% and a purity of 95...
Embodiment 2
[0038] Add the compounds of the above formulas (II), (III) and (IV) and cuprous iodide (CuI) into acetonitrile, then raise the temperature to 60° C., and stir and seal the reaction at this temperature for 8 hours.
[0039] Wherein, the molar ratio of formula (II) compound and cuprous iodide (CuI) is 1:0.2; The molar ratio of formula (II) compound and (III), (IV) compound is 1:1.2:1.2; The ratio of the compound of formula (II) in moles (mmol) to acetonitrile in milliliters (ml) is 1:5.
[0040] After the reaction was complete, the reaction system was naturally cooled to room temperature, and the solvent was distilled off under reduced pressure to obtain a crude product. The crude product was subjected to 200-300 mesh silica gel column chromatography, using a mixture of ethyl acetate and petroleum ether as an eluent, wherein The volume ratio of ethyl acetate and sherwood oil is 1:4, thereby obtains the target product formula (I) compound (C 20 h 22 N 2 o 3 S) with a yield of...
Embodiment 3
[0045] Add the compounds of formulas (II), (III) and (IV) and copper iodide (CuI) to acetonitrile, then raise the temperature to 40° C., and stir and seal the reaction at this temperature for 12 hours.
[0046] Wherein, the molar ratio of formula (II) compound and cuprous iodide (CuI) is 1:0.05; The molar ratio of formula (II) compound and (III), (IV) compound is 1:2.5:2.5; The ratio of the compound of formula (II) in moles (mmol) to acetonitrile in milliliters (ml) is 1:2.
[0047] After the reaction was complete, the reaction system was naturally cooled to room temperature, and the solvent was distilled off under reduced pressure to obtain a crude product. The crude product was subjected to 200-300 mesh silica gel column chromatography, using a mixture of ethyl acetate and petroleum ether as an eluent, wherein The volume ratio of ethyl acetate and sherwood oil 1:6, thus obtain the target product formula (I) compound (C 19 h 19 ClN 2 o 3 S) with a yield of 75% and a purit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com