Preparation method of heterogeneously catalyzed 2-trifluoromethyl substituted benzimidazole compound
A technology of trifluoromethyl and benzimidazole, which is applied in the field of preparation of benzimidazole compounds, can solve the problems of unreported synthesis methods, etc., and achieve the effects of strong designability, simple post-treatment, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] Catalyst preparation:
[0030] carbon nitride (g-C 3 N 4 ) was dispersed in DMF for three hours, and then ultrasonically treated carbon nitride, isonicotinic acid chloride hydrochloride and a few drops of triethylamine were mixed in DMF and reacted at 60°C for 6 hours. The reaction solution is centrifuged, washed and freeze-dried to obtain the complex of carbon nitride and isonicotinic acid chloride (g-C 3 N 4 -INCH). will g-C 3 N 4 -INCH and copper sulfate pentahydrate were mixed in the aqueous solution, triethylamine was added dropwise and reacted at 60°C for 3 hours. After the reaction is complete, the product is washed several times with DMF and ultrapure water, and freeze-dried to obtain a copper-doped carbon nitride catalyst (Cu / g-C 3 N 4 ).
[0031] Measurement of Cu / g-C using inductively coupled plasma optical emission spectrometer (ICP-OES) 3 N 4 The content of copper in the medium is 16%, and the transmission electron microscope (TEM) test Cu / g-C 3...
Embodiment 1~10
[0033] Add copper-doped carbon nitride, potassium carbonate, trifluoroethylimidoyl chloride (II), amine (III) and 2 mL of organic solvent into a 35 mL Schlenk tube according to the raw material ratio in Table 1, mix and stir evenly, and The reaction conditions of table 2 were reacted for 18-30 hours, filtered, mixed with silica gel, and purified by column chromatography to obtain the corresponding 2-trifluoromethyl-substituted benzimidazole compound (I). The reaction process is shown in the following formula:
[0034]
[0035] Table 1 The raw material addition of embodiment 1~10
[0036]
[0037] Table 2
[0038]
[0039] In Table 1 and Table 2, T is the reaction temperature, t is the reaction time, Ph is phenyl, Me is methyl, i-Pr is isopropyl, n-Bu is n-butyl, n-Amyl is n-pentyl , Bn is benzyl, CF 3 It is trifluoromethyl, and DMF is N,N-dimethylformamide.
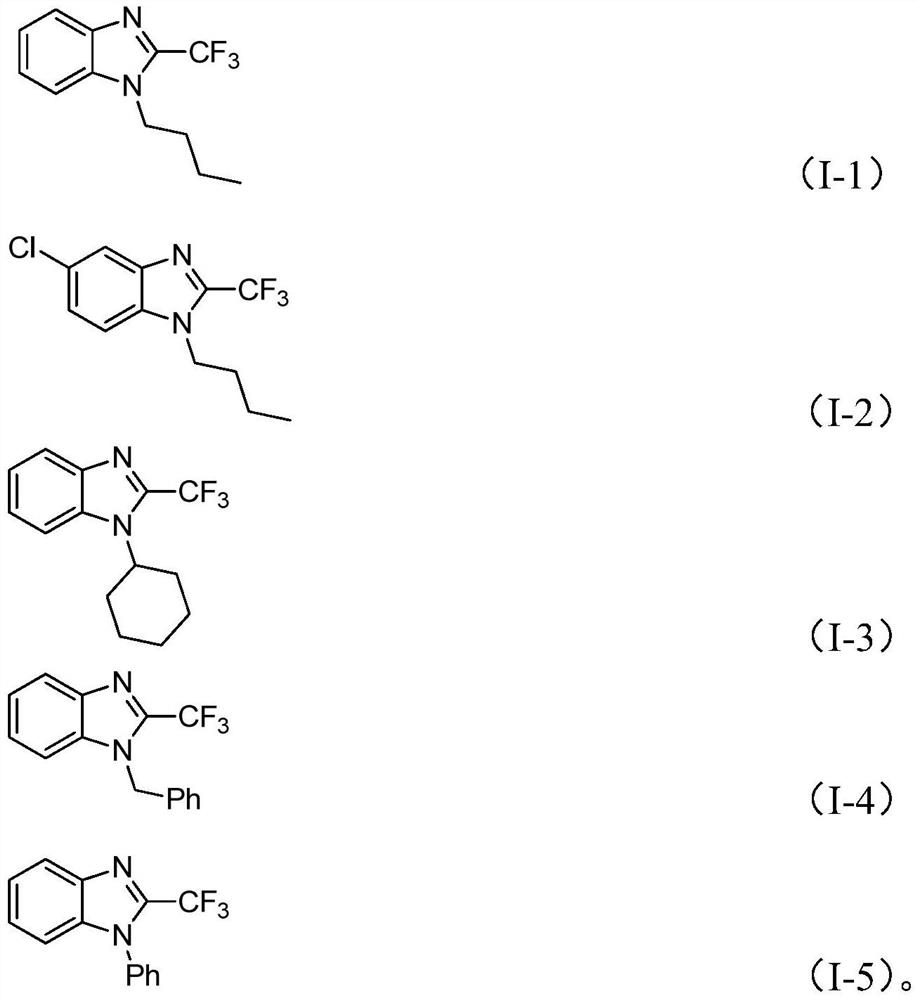
[0040] The structural confirmation data of the compounds prepared in Examples 1-5:
[0041] The nuclear ma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com