New application of algin in treatment of hepatolenticular degeneration
A technology of hepatolenticular degeneration and alginate, applied in the direction of medical raw materials derived from algae, medical preparations containing active ingredients, organic active ingredients, etc., can solve the problem that the effect cannot be satisfied and it is difficult to fundamentally prevent the disease Issues such as development and reversal of disease progression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: The preparation technology of alginate.
[0041] The preparation process of alginate is as follows. The raw material of brown algae is one or more of kelp, wakame, ascophyllum, sargassum or macroalgae. After soaking, chopping, digesting, diluting, filtering, washing, calcium precipitation, hydrochloric acid It is prepared through the processes of decalcification, alkali dissolution, ethanol precipitation, filtration, drying, pulverization, and finished product.
[0042] The preparation process of alginate comprises the following steps:
[0043] (1) Soak and wash the brown algae with water, and then mix them evenly with an alkali agent to obtain a paste-like alginate; the soaking time is 2 hours, and the water consumption is 8 times the quality of the brown algae; the alkali agent includes sodium carbonate and sodium hydroxide , the dosage of the alkali agent is 25% of the quality of the brown algae, the digestion temperature is 60°C, and the digestion tim...
Embodiment 2
[0049] Example 2: Alginate can significantly promote the discharge of copper ions in the body.
[0050] What this embodiment adopts is the alginate obtained in embodiment 1.
[0051] 1. Seventy adult healthy male Kunming mice were provided by Jinan Pengyue Experimental Animal Breeding Company, SCXK (Lu) 20190003. The weight of mice is 25g~30g, SPF grade. Before the experiment, the animals were placed in an animal laboratory at 25±2°C for one week of adaptive feeding, with free access to food and water, and 12h / 12h natural light. Randomly select 10 rats as the control group and feed them with common feed. The other 60 mice were established with HLD model by copper sulfate loading diet. After 7 days of adaptive feeding, feed the feed containing copper sulfate (1g / kg) and the water of (0.185%) copper sulfate, after feeding continuously for 28 days, put the mice in metabolic cages, keep urine for 24 hours continuously, apply induction Copper content in urine by coupled plasma ...
Embodiment 3
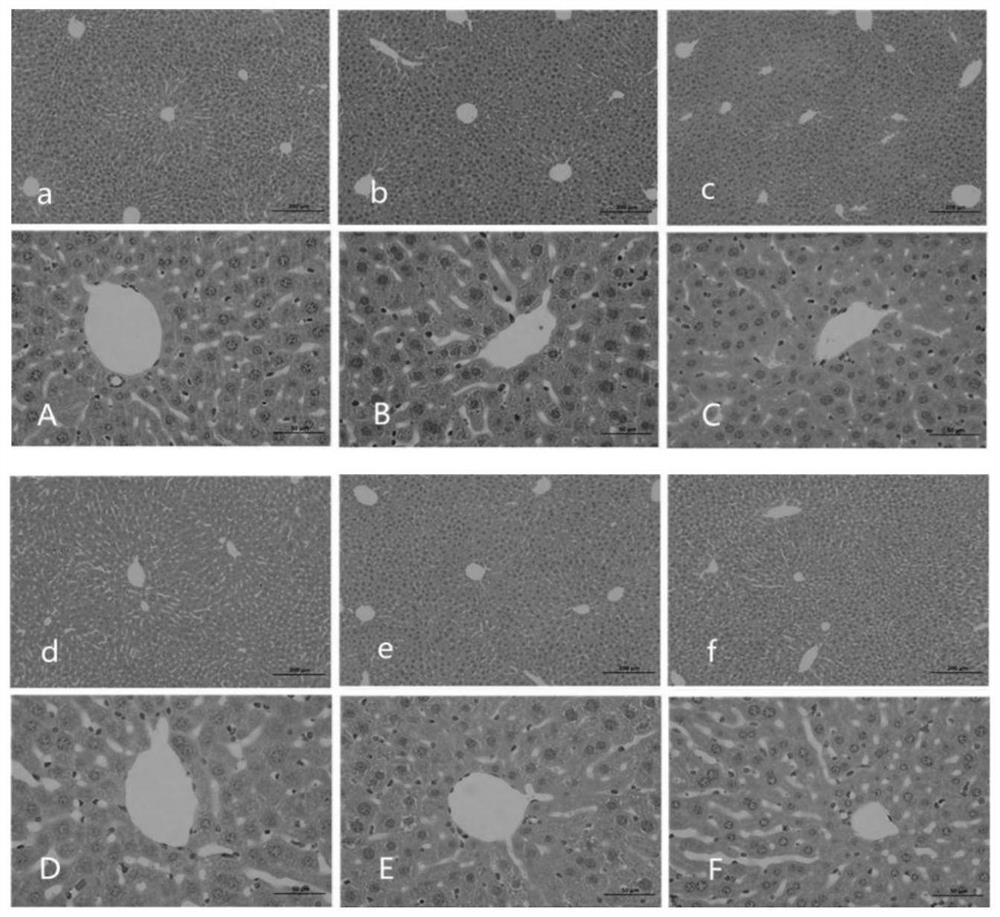
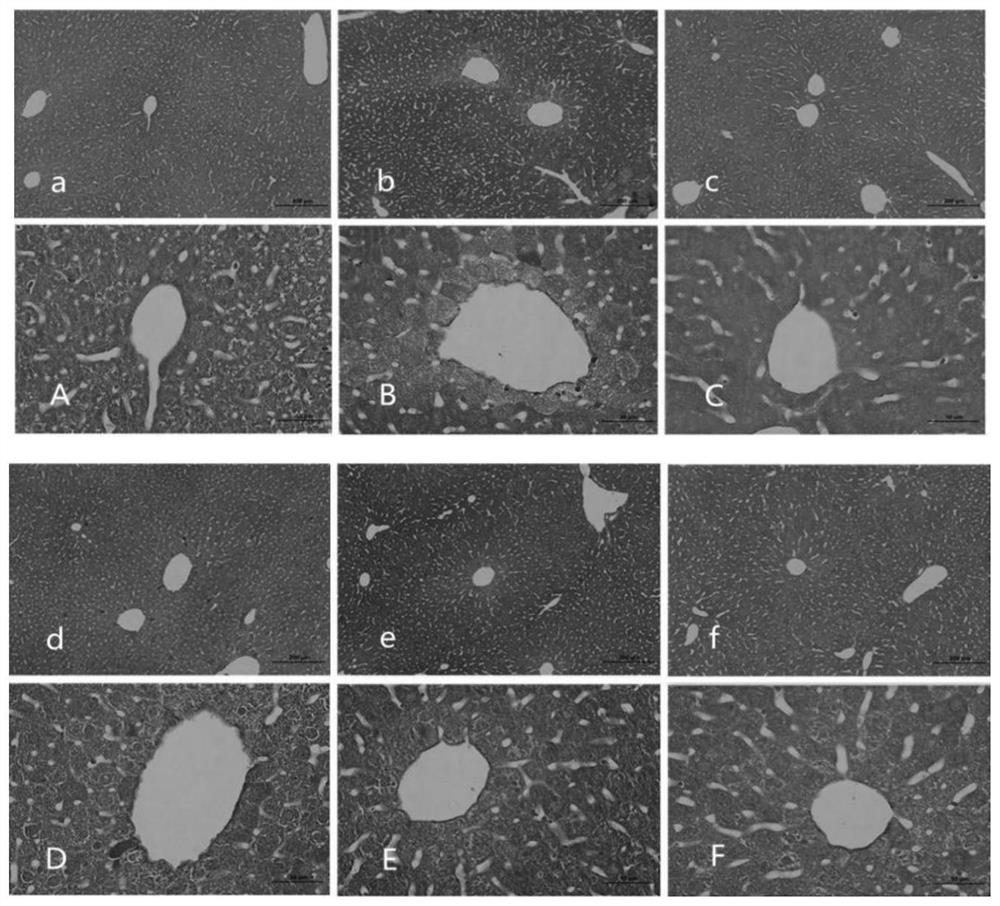
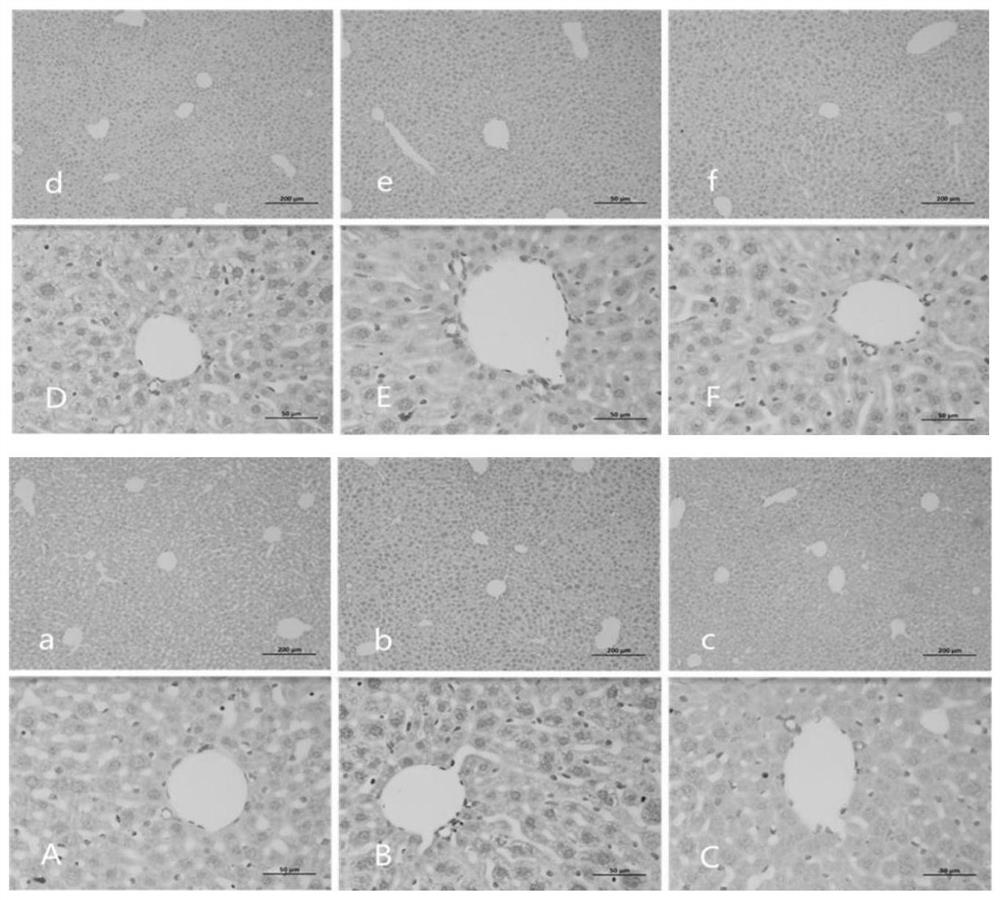
[0065] Example 3: Alginate can improve liver function.
[0066] What this embodiment adopts is the alginate obtained in embodiment 1.
[0067] What this embodiment adopts is the data analysis obtained in embodiment 2.
[0068] After intervening with alginate for 28 days, 5 mice were taken from each group of control group, model group, positive drug control group, low-dose treatment group, middle-dose treatment group and high-dose treatment group, and injected intraperitoneally with 10% chloral hydrate (300mg / kg) anesthetize the mouse, take 1mL of blood through the heart, let it stand for 10min, centrifuge at 4000rpm for 10min, and collect the serum.
[0069] (1) ALT and AST activity: take 50 μL of the above-mentioned stored serum samples, and use a CS-800 automatic biochemical analyzer to detect liver function indicators: alanine aminotransferase (ALT, U / L), aspartate amino Transferase (AST, U / L), total protein (TP, g / L), albumin (ALB, g / L). After modeling, the activities o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com