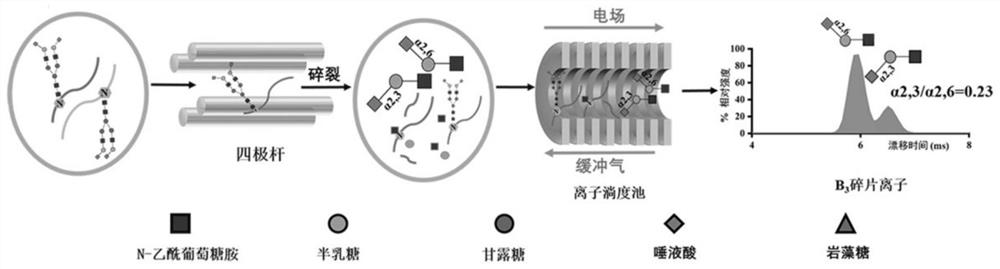
Relative quantification method of N-glycopeptide terminal sialic acid alpha 2, 6 and alpha 2, 3 connection isomerism
A terminal sialic acid, relative quantification technique, applied in the field of analytical chemistry, can solve problems such as the inability to use glycopeptide sialic acid α2, limited analysis of polysaccharide peptide samples, lack of convenient, fast, and environment-friendly quantitative methods, etc., and achieve high accuracy performance, optimized environment, and high quantitative accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] All chemical reagents and solvents used in the examples are analytically pure.
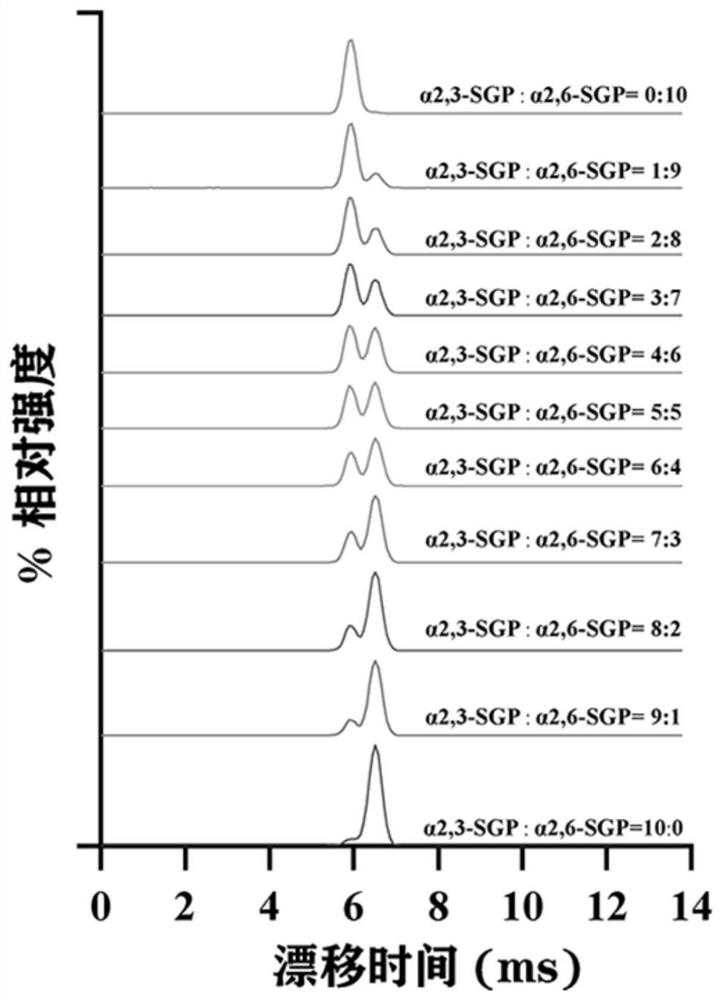
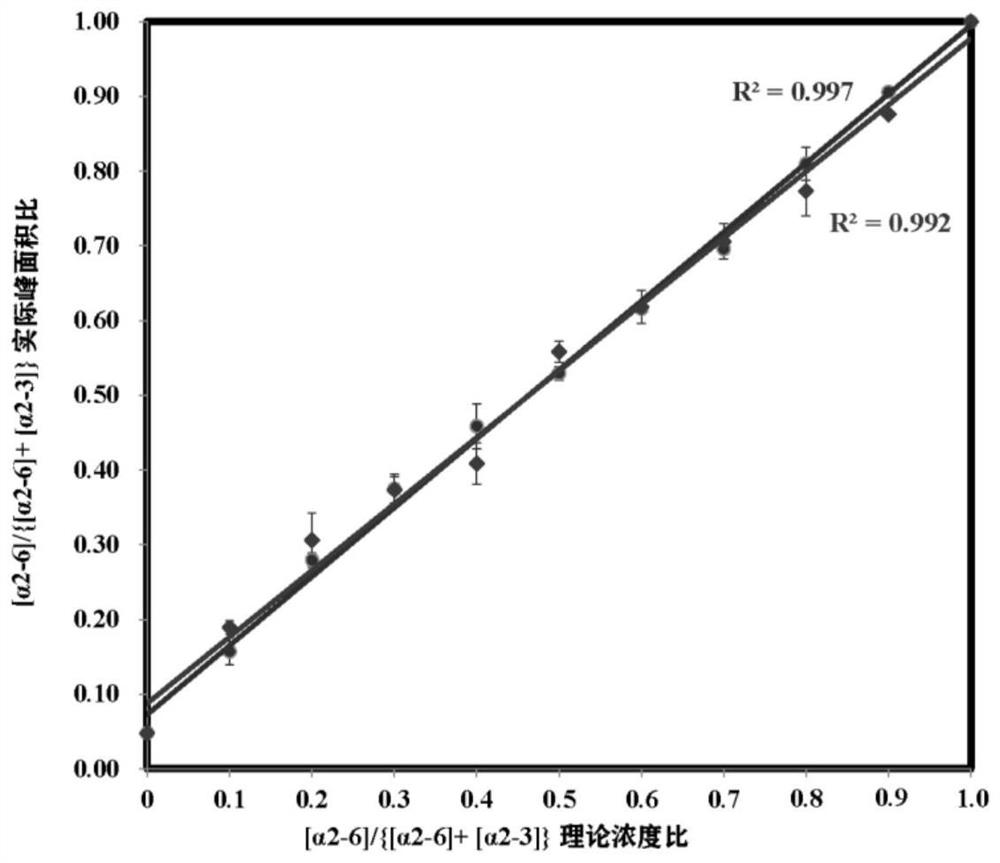
[0023] (1) Standard N-glycopeptide sample preparation: Dissolve standard intact N-glycopeptides (α2,6-SGP and α2,3-SGP) linked to terminal sialic acid α2,6 and α2,3 in 0.1 % TFA to obtain two standard stock solutions at a final concentration of 8.7 μM. Standard glycopeptides α2,6-SGP and α2,3-SGP were mixed in absolute concentration (87 nM) in different mixing ratios (0, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, 1) , prepared as a sample for analysis.
[0024] (2) Liquid phase separation of mixed standard glycopeptides: On-line LC uses the M-Class nano-LC system of Waters Company: Phase A is 0.1% FA aqueous solution; phase B is ACN solution with 0.1% TFA. N-glycopeptides were loaded on-column at a flow rate of 300 nL / min for 4 min. Then it enters the analytical column for separation, and the chromatographic column adopts 15 cm C 18 Column (nanoE MZ PST CSH130 C 18 1.7μ75μm × 150mm...
Embodiment 2
[0030] Using haptoglobin (Hp) purified in serum as an analyte, the complete N- Relative proportion of α2,3 and α2,6 isomeric linkages of glycopeptide terminal sialic acids.
[0031] (1) Purification of serum haptoglobin: Serum samples were centrifuged at 12000*g for ten minutes, and then purified using HiTrap columns. The haptoglobin bound to the column was eluted with elution buffer (pH 3.0, 100 mM Glycine, 0.5 M NaCl), followed by acetone precipitation and concentration.
[0032] (2) Enzymatic hydrolysis of haptoglobin and purification of N-glycopeptide: Take 190 μl of haptoglobin with a concentration of 1 mg / mL, add 10 μl of 200 mM dithiothreitol (DTT) solution, react at 56 °C for 1 h, and perform protein Denaturation and reduction treatment. Then add 10ul of 400mM iodoacetamide solution (IAA), and react in the dark for 30 min at room temperature to carry out the alkylation treatment of the protein. Finally, 4 μg of trypsin was added for enzymatic hydrolysis of the prote...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com