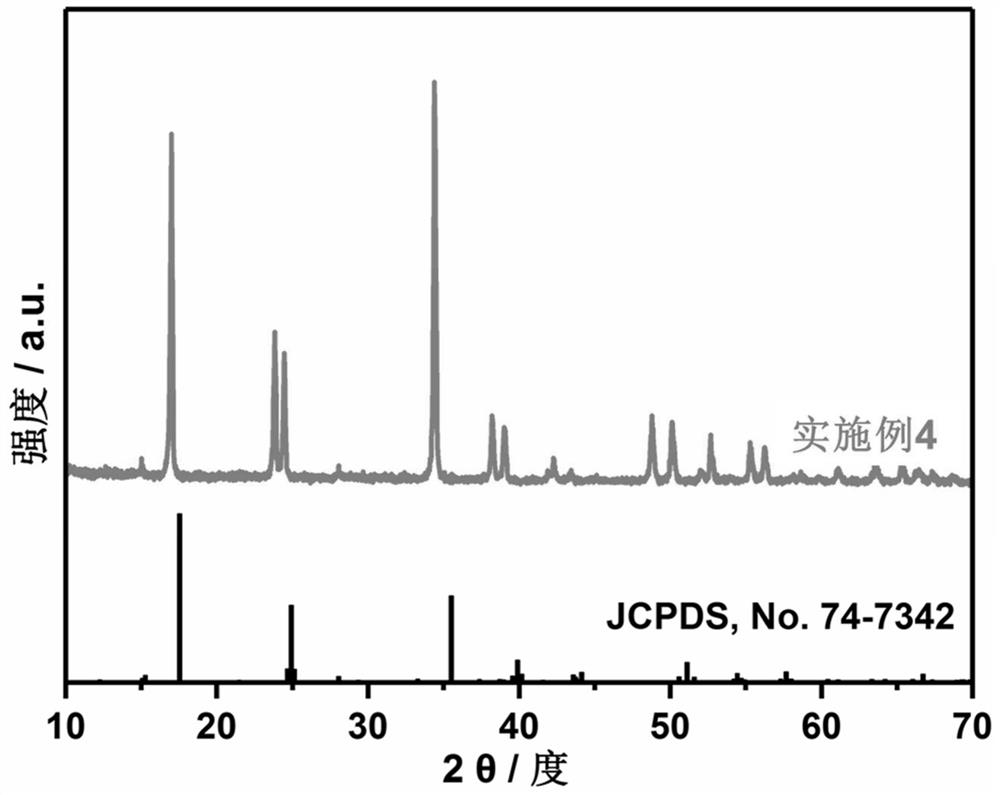
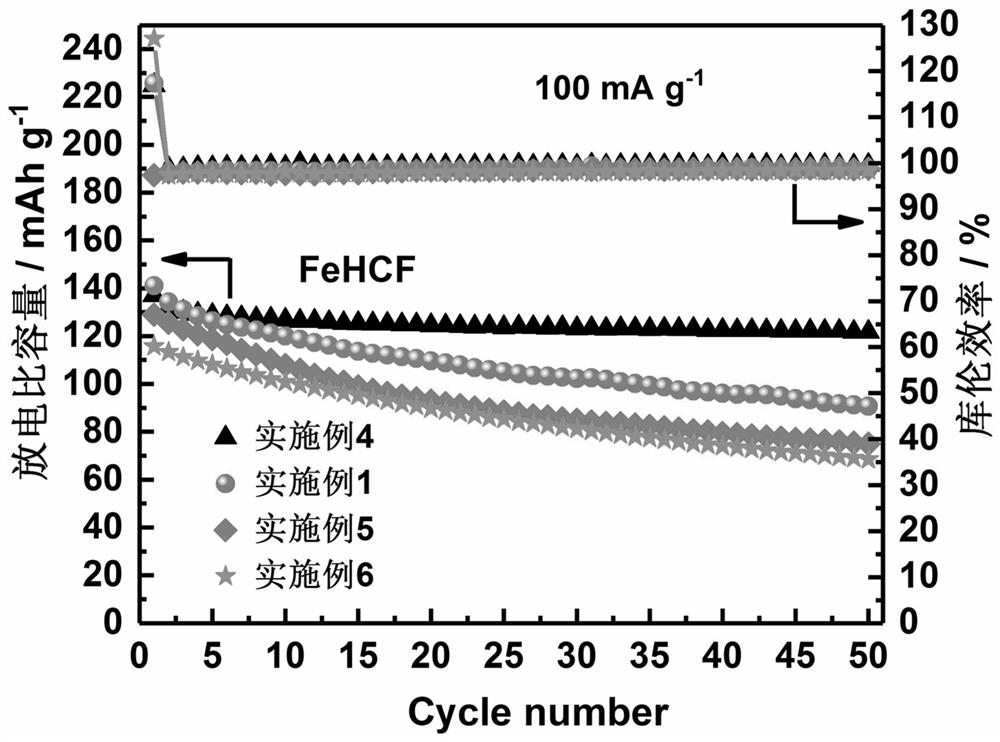
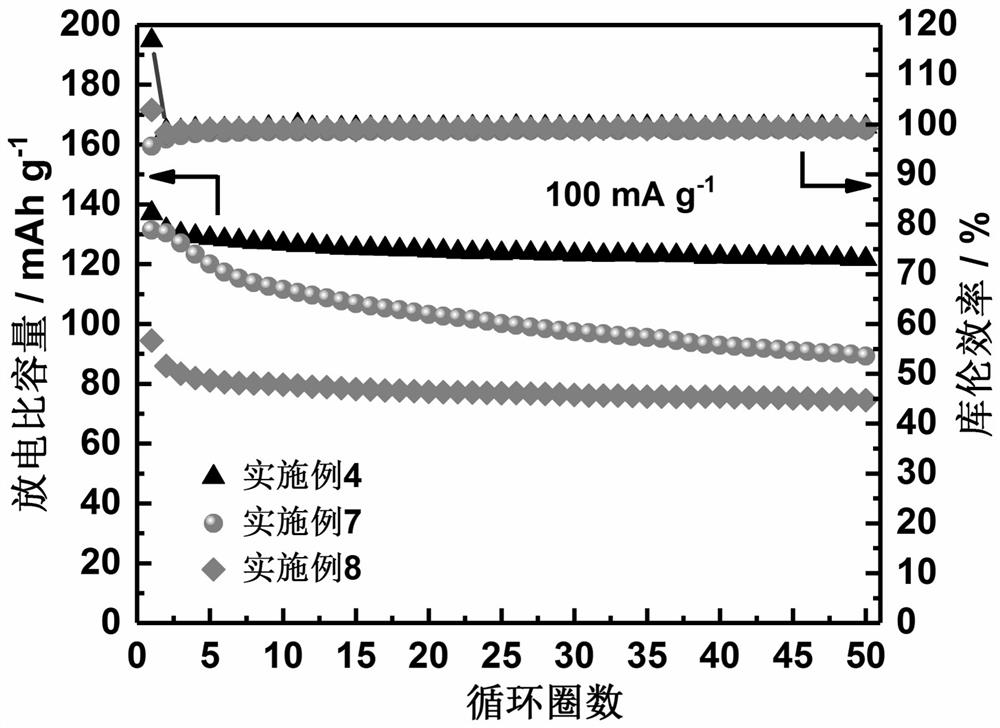
Prussian blue sodium ion battery positive electrode material and preparation method thereof
A sodium-ion battery, Prussian blue technology, applied in the field of preparation of Prussian blue-like materials by slow co-precipitation method, can solve the problems of low specific capacity and unsatisfactory cycle performance, achieve high capacity, slow down crystallization rate, and simplify production process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 5 mmol FeSO 4 ·7H 2 O and C 4 H 6 O 6 Dissolve 25 mmol in 50 ml of deionized water to form solution A, 5 mmol Na 4 Fe(CN) 6 ·10H 2 O and 1 g C 6 H 8 O 6 Dissolved in 50 ml of deionized water to form solution B; 1 g of PVP and 6 g of NaCl were dissolved in deionized water to form solution C; solution A and solution B were simultaneously added dropwise to solution C at a rate of 0.167 ml / min, while dropping Stir and heat until the dropwise addition is completed, the solution becomes a white suspension, continue to stir for 12 h, and then age for 24 h; then centrifuge with deionized water and absolute ethanol on a centrifuge with a rotation speed of ≧8000 rpm / min. Washed three times; finally, the dark blue solid was dried in a vacuum oven at 120 °C for 24 h to obtain the target product iron-based Prussian blue cathode material, marked as Na 2 FeFe(CN) 6 -1. The resulting Na 2 FeFe(CN) 6 -1 The positive electrode material is stirred with acetylene black and po...
Embodiment 2
[0034] 5 mmol Mn(Ac) 2 ·4H 2 O and 50 mmol C 4 H 6 O 6 Dissolve in 50 ml of deionized water to form solution A, 5 mmol Na 4 Fe(CN) 6 ·10H 2 O and 1 g C 6 H 8 O 6 Dissolved in 50 ml of deionized water to form solution B; 1 g of PVP and 6g of NaCl were dissolved in deionized water to form solution C; solution A and solution B were simultaneously added dropwise to solution C at a rate of 0.167 ml / min, while dropping Stir and heat until the dropwise addition is completed, the solution turns into a white suspension, continue to stir for 12 h, and then age for 24 h; Centrifuged and washed three times; finally, the obtained precipitate was dried in a vacuum oven at 120 °C for 24 h to obtain the target product manganese-based Prussian blue cathode material, marked as Na 2 MnFe(CN) 6 -1. The resulting Na 2 MnFe(CN) 6 -1 The positive electrode material is stirred with acetylene black and polyvinylidene fluoride (PVDF) to form a slurry, which is coated on aluminum foil, dri...
Embodiment 3
[0036] 5 mmol Co(Ac) 2 ·4H 2 O and 25 mmol C 4 H 6 O 6 Dissolve in 50 ml of deionized water to form solution A, 5 mmol Na 4 Fe(CN) 6 ·10H 2 O and 1 g C 6 H 8 O 6 Dissolved in 50 ml of deionized water to form solution B; 1 g of PVP and 6g of NaCl were dissolved in deionized water to form solution C; solution A and solution B were simultaneously added dropwise to solution C at a rate of 0.167 ml / min, while dropping Stir and heat until the dropwise addition is completed, the solution turns into a white suspension, continue to stir for 12 h, and then age for 24 h; Centrifuged and washed three times; finally, the obtained precipitate was dried in a vacuum oven at 120 °C for 24 h to obtain the target product cobalt-based Prussian blue cathode material, marked as Na 2 CoFe(CN) 6 -1. The resulting Na 2 CoFe(CN) 6 -1 The positive electrode material is stirred with acetylene black and polyvinylidene fluoride (PVDF) to form a slurry, which is coated on aluminum foil, dried,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com