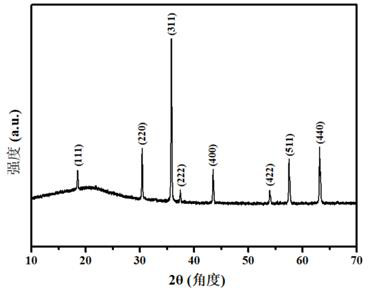
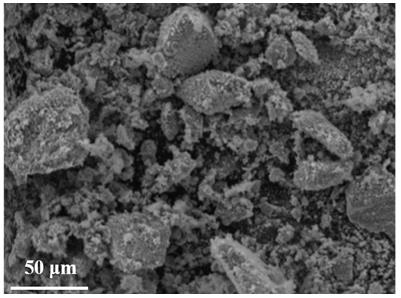
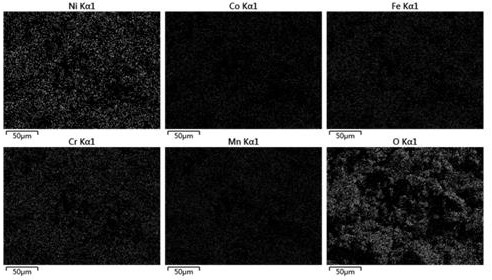
Method for synthesizing spinel type high-entropy oxide material (MCoFeCrMn)3O4 by hydrothermal method
A spinel-type, hydrothermal technology, applied in the field of infrared heating and solar energy absorption, can solve the problem that high-entropy oxide materials have not been retrieved, and achieve the effects of alleviating the energy crisis, mild reaction conditions and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1, (NiCoFeCrMn) 3 o 4 Preparation and properties of high-entropy oxides
[0028]Weigh 1.7563 g (0.006 mol) of nickel nitrate, 1.7457 g (0.006 mol) of cobalt nitrate, 2.4243 g (0.006 mol) of iron nitrate, 2.4539 g (0.006 mol) of chromium nitrate and 2.1653 g (0.006 mol) of manganese nitrate, and dissolve them in Stir evenly in 12mL ultrapure water, mix the five metal salt solutions and continue to stir until completely mixed to obtain a mixed solution of metal nitrate; then weigh 2.5438g (0.024 mol) of sodium carbonate, add it to the above mixed solution and stir evenly; Then the mixed solution was transferred to a polytetrafluoroethylene-lined stainless steel reactor, placed in a blast oven at 150 °C for 4 h, and then cooled to room temperature; the reaction solution was filtered under reduced pressure and washed 5 times with ultrapure water, The precipitate was separated by suction filtration and dried to obtain a solid powder; finally, the obtained solid ...
Embodiment 2
[0030] Embodiment 2, (NiCoFeCrMn) 3 o 4 Preparation and properties of high-entropy oxides
[0031] Weigh 1.7563 g (0.006 mol) of nickel nitrate, 1.7457 g (0.006 mol) of cobalt nitrate, 2.4243 g (0.006 mol) of ferric nitrate, 2.4539 g (0.006 mol) of chromium nitrate and 2.1653 g (0.006 mol) of manganese nitrate were dissolved in 60mL Stir evenly in ultrapure water, mix the five metal salt solutions and continue to stir until completely mixed to obtain a mixed solution of metal nitrate; then weigh 1.2719 g of sodium carbonate (0.012 mol) and add it to the above mixed solution and stir evenly; then The above mixed solution was transferred to a polytetrafluoroethylene-lined stainless steel reaction kettle and placed in a blast oven at 120°C for heat preservation for 1 h; after cooling to room temperature, the reaction solution was filtered under reduced pressure and washed with ultrapure water for 3 times, and then separated by suction filtration. Precipitate and dry to obtain s...
Embodiment 3
[0033] Embodiment 3, (NiCoFeCrMn) 3 o 4 Preparation and properties of high-entropy oxides
[0034] Weigh 1.7563 g (0.006 mol) of nickel nitrate, 1.7457 g (0.006 mol) of cobalt nitrate, 2.4243 g (0.006 mol) of iron nitrate, 2.4539 g (0.006 mol) of chromium nitrate and 2.1653 g (0.006 mol) of manganese nitrate were dissolved in 10mL Stir evenly in ultrapure water, mix the five metal salt solutions and continue to stir until completely mixed to obtain a mixed solution of metal nitrate; then weigh 3.8156 g (0.036 mol) of sodium carbonate and add it to the above mixed solution and stir evenly; then The above mixed solution was transferred to a polytetrafluoroethylene-lined stainless steel reactor and placed in a blast oven at 180 °C for heat preservation for 7 hours. After cooling to room temperature, the reaction solution was filtered under reduced pressure and washed 7 times with ultrapure water, and separated by suction filtration. Precipitate and dry to obtain a solid powder;...
PUM
| Property | Measurement | Unit |
|---|---|---|
| emissivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com