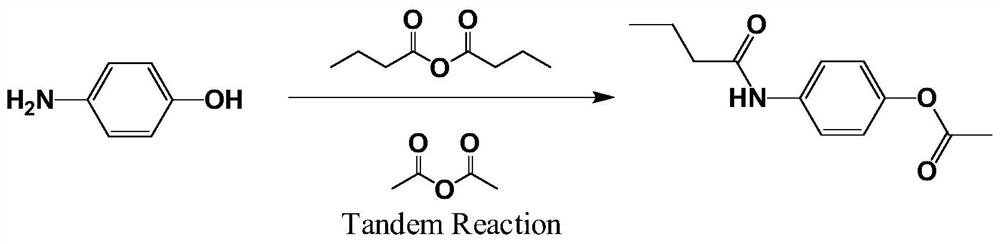
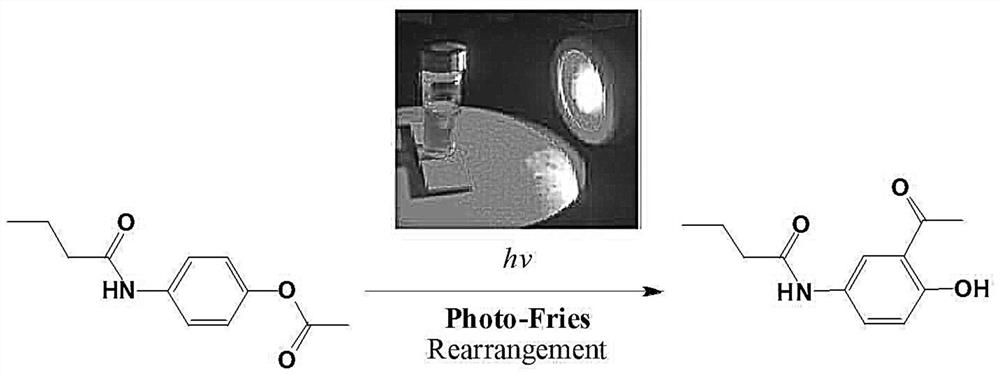
Beta receptor blocker Acebutolol intermediate synthesized by photochemical Fries rearrangement
A photochemical and β-receptor technology, which is applied in the fields of organic photochemistry and medicinal chemistry, can solve problems such as high cost, high toxicity and pollution, and complex reaction operations, and achieve the effects of low pollution, simple and easy synthesis methods, and simple purification operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 1.1. Put 2.64g of n-butyric anhydride, 1.09g of p-aminophenol, and 3.40g of toluene into the reaction flask, heat up and reflux for dehydration. Reflux for about 9 hours, (moisture content is about 1.45g) carry out atmospheric distillation and reclaim toluene. After recovering about 80% of the amount of toluene, carry out vacuum distillation to evaporate the toluene. Cool to T<35°C, add 10 g of water, stir and dropwise add 6.00 g of sodium hydroxide, and then dropwise add 2.20 g of acetic anhydride. Stir for 3.5 hours and filter. Recrystallized from acetone. Activated carbon decolorization, hot filtration. The crystalline product was dried and weighed to obtain 1.43 g of N-(4-acetoxyphenyl)butyramide, with a yield of 65.1%.
[0027] 1.2. Put 1.1 g of the intermediate product N-(4-acetoxyphenyl) butyramide in the above step 1.1 into a photoreaction container and add 5.5 g of tetrahydrofuran, and after fully dissolving, use ultraviolet-visible light to irradiate the p...
Embodiment 2
[0029] 2.1. Put 2.85g of n-butyric anhydride, 1.09g of p-aminophenol, and 3.40g of toluene into the reaction flask, heat up and reflux for dehydration. Reflux for about 8 hours, (moisture content is about 1.45g) carry out atmospheric distillation and reclaim toluene. After recovering about 80% of the amount of toluene, carry out vacuum distillation to evaporate the toluene. Cool to T<35°C, add 20g of water, stir and dropwise add 6.00g of potassium hydroxide, and then dropwise add 2.30g of acetic anhydride. Stir for 3.5 hours and filter. Recrystallized from acetone. Activated carbon decolorization, hot filtration. The crystalline product was dried and weighed to obtain 1.23 g of N-(4-acetoxyphenyl)butyramide, with a yield of 61.3%.
[0030] 2.2. Put 1.1 g of the intermediate product N-(4-acetoxyphenyl) butyramide in the above step 2.1 into a photoreaction container and add 5.5 g of tetrahydrofuran, fully dissolve and irradiate the photoreaction container with ultraviolet-vi...
Embodiment 3
[0032] 3.1. Put 3.15g of n-butyric anhydride, 1.09g of p-aminophenol, and 3.40g of toluene into the reaction bottle, and heat up and reflux for dehydration. Reflux for about 10 hours, (moisture content is about 1.45g) carry out atmospheric distillation and reclaim toluene. After recovering about 81% of the amount of toluene, carry out vacuum distillation to evaporate the toluene. Cool to T<35°C, add 20 g of water, stir and add 6.00 g of potassium hydroxide dropwise, and then add dropwise 2.70 g of acetic anhydride. Stir for 4.5 hours and filter. Recrystallized from acetone. Activated carbon decolorization, hot filtration. The crystalline product was dried and weighed to obtain 1.47 g of N-(4-acetoxyphenyl)butyramide, with a yield of 73.2%.
[0033] 3.2. Put 1.1 g of the intermediate product N-(4-acetoxyphenyl) butyramide in the above step 3.1 into the photoreaction container and add 6.6 g of tetrahydrofuran, and after fully dissolving, use ultraviolet-visible light to irra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com