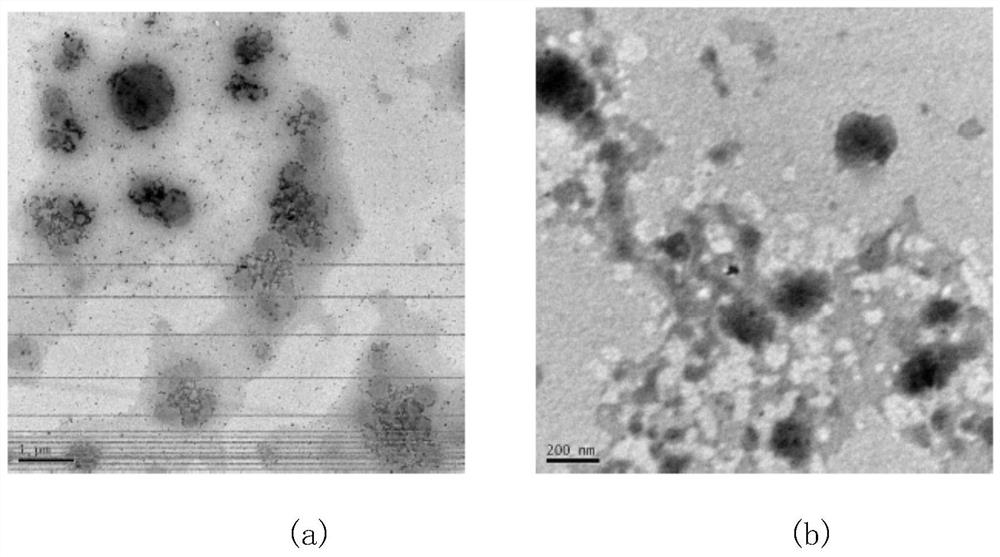
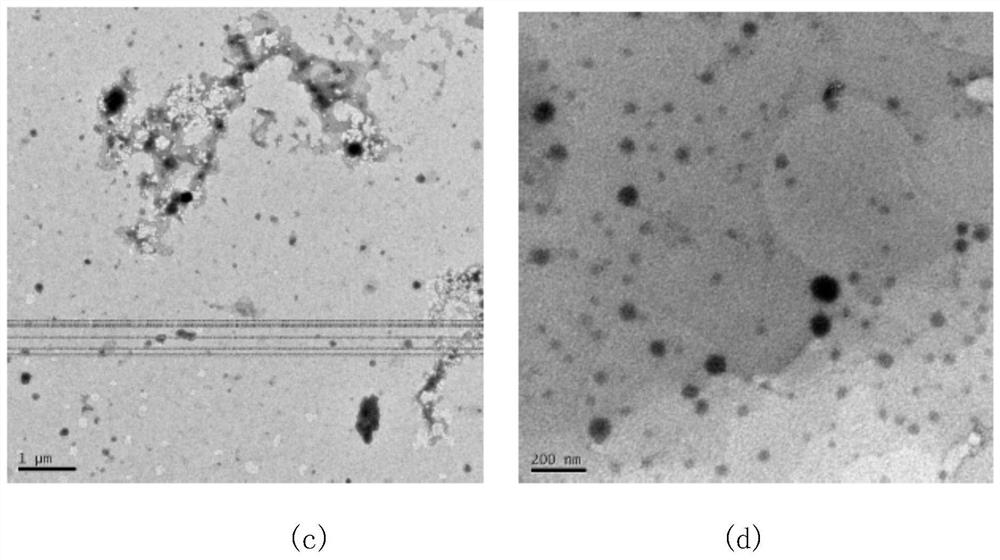
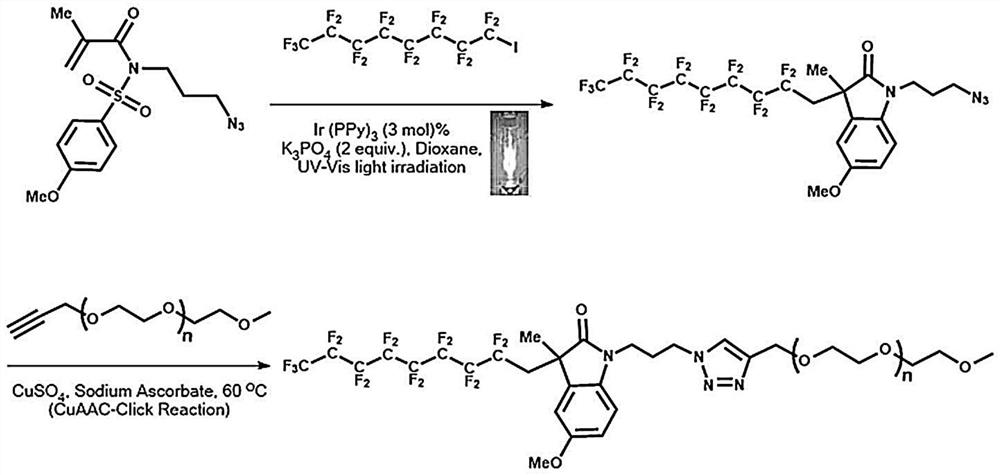
Nitrogen-containing heterocyclic fluorocarbon surfactant synthesized by adopting photoreaction-click reaction method and preparation of water-phase micelle of nitrogen-containing heterocyclic fluorocarbon surfactant
A click reaction and fluorocarbon surface technology is applied in the field of organic photochemistry to achieve the effects of simple and easy synthesis method, simple preparation, and simple purification operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028]The first step is to invest in N- (3-lamnopropyl) -N- (for methoxy sulfonyl) methacrylamide (676 mg, 0.002 mol) and 1-iodide perfluoroctane. (1416 mg, 0.0013 mol), an optical catalyst IR of a molar ratio of N- (3-azide-propylene) -N- (for methoxy sulfonyl) methacrylamide is added to a photocatalytic IR of 3% (38 mg, 0.00006 mol) (PPY) 3 And the molar ratio of N- (3-azide-propylene) -N- (for methoxysulfonyl) methacrylamide is 2 (830 mg, 0.002 mol) of potassium phosphate K 3 PO 4 , 15 ml of the organic solvent dioxane (Dioxane) was added as a solvent, and an ultraviolet-visible light was used as a photolraction container and stirred for 24 h, and a photochemical molecule was closed, and the reaction product was subjected to silica gel column chromatography isolation purification (eluent ethyl acetate : Petroleum ether volume ratio V: v = 5: 1) Butketate (860 mg, 62%). The ultraviolet-visible light source is Bluewave15W / CM 2 LED Prime Uva Spot Curing System, Penang, Malaysi...
Embodiment 2
[0035] The first step is to invest N- (3-laminated nitrogen-based) -N- (methoxy sulfonyl) methacrylamide (338 mg, 0.001 mol) and 1-iodide perfluoroctane. (708 mg, 0.0013 mol), an optical catalyst IR of a molar ratio of N- (3-azide-propylene) -N- (for methoxy sulfonyl) methacrylamide is added. (PPY) 3 And the molar ratio of N- (3-azide-propylene) -N- (for methoxyulfonyl) methacrylamide is 2 (415 mg, 0.002 mol) of potassium phosphate K 3 PO 4 The 7 ml of the organic solvent dioxane (Dioxane) was added as a solvent, and the ultraviolet-visible light was used as a photoreactive reaction vessel and stirred for 24 h, and the inner closed loop reaction in photochemical molecules was carried out, and the reaction product was subjected to silica gel column chromatography isolation purification (eluent ethyl acetate : Petroleum ether volume ratio V: v = 5: 1) Butketate (402 mg, 58%). The ultraviolet-visible light source is Bluewave15W / CM 2 LED Prime Uva Spot Curing System, Penang, Malay...
Embodiment 3
[0042] The first step is to invest in N- (3-lamnopropyl) -N- (for methoxy sulfonyl) methacrylamide (676 mg, 0.002 mol) and 1-iodide perfluoroctane. (1416 mg, 0.0013 mol), an optical catalyst IR of a molar ratio of N- (3-azide-propylene) -N- (for methoxy sulfonyl) methacrylamide is added to a photocatalytic IR of 3% (38 mg, 0.00006 mol) (PPY) 3 And the molar ratio of N- (3-azide-propylene) -N- (for methoxysulfonyl) methacrylamide is 2 (830 mg, 0.002 mol) of potassium phosphate K 3 PO 4 , 15 ml of the organic solvent dioxane (Dioxane) was added as a solvent, and an ultraviolet-visible light was used as a photolraction container and stirred for 24 h, and a photochemical molecule was closed, and the reaction product was subjected to silica gel column chromatography isolation purification (eluent ethyl acetate : Petroleum ether volume ratio V: v = 5: 1) Butketate (943 mg, 68%). The ultraviolet-visible light source is Bluewave15W / CM 2 LED Prime Uva Spot Curing System, Penang, Malays...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface tension | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com