Preparation method of quinoline TGF-beta1 inhibitor
A catalyst and solvate technology, applied in the field of medicinal chemistry, can solve the problems of low target selectivity and specificity, off-target toxic and side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
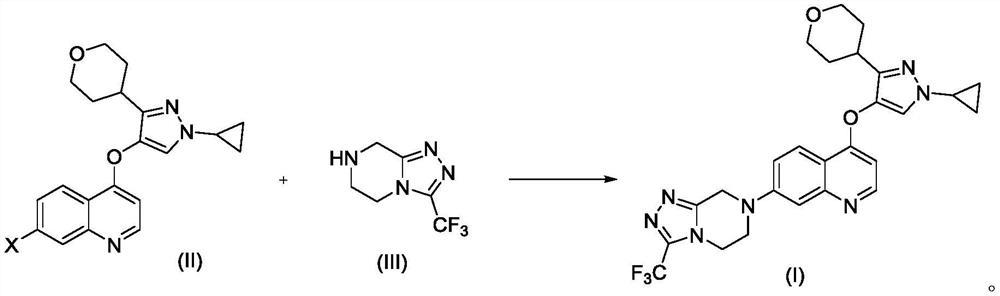
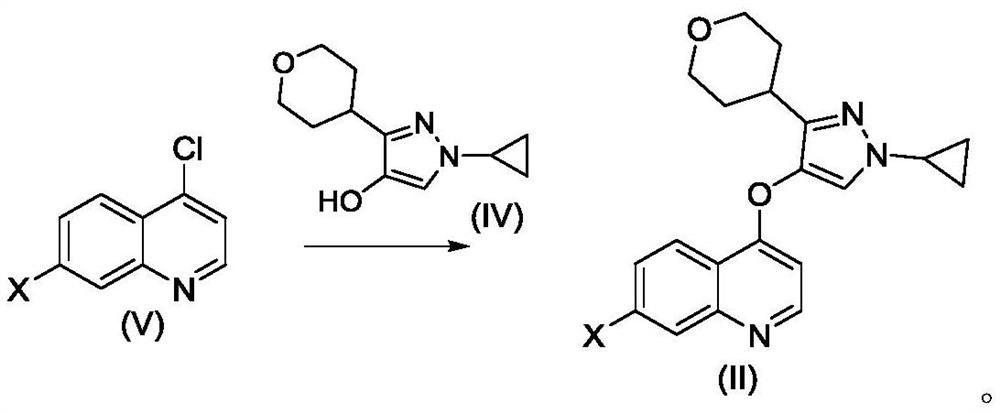
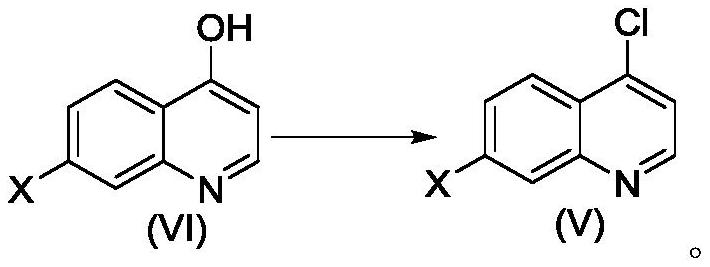
[0070] Example 1 4-((1-cyclopropyl-3-(tetrahydro-2H-pyran-4-yl)-1H-pyrazol-4-yl)oxy)-7-(3-(trifluoro Preparation of methyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl)quinoline
[0071]
[0072] Step 1: Preparation of 2-bromo-1-(tetrahydro-2H-pyran-4-yl)ethan-1-one
[0073]
[0074] Under nitrogen protection, add methanol (100mL) and 1-(tetrahydro-2H-pyran-4-yl)ethanone (20.0g, 156mmol) in sequence to a 1000mL three-necked flask, cool down to below -15°C, and slowly drop Enter liquid bromine and keep the temperature below -15°C. After dropping, raise the temperature to 0°C, react for 45 minutes, then raise the temperature to 10°C, react for 45 minutes, keep the internal temperature below room temperature and slowly add 11mol / L sulfuric acid (55mL) dropwise, and react overnight at room temperature. Monitor the completion of the reaction, add ethyl acetate and sodium chloride aqueous solution for extraction, combine the organic layers, adjust the pH value of the ...
experiment example 1
[0109] Experimental example 1 compound in vitro ALK5 kinase activity evaluation
[0110] 1. Experimental materials
[0111] 1.1 Compounds
[0112] The compound of formula (I) of the present invention in Example 1 was prepared to 10 mM with DMSO, and then diluted to 3.333 μM, 1.111 μM, 370 nM, 123 nM, 41 nM, 14 nM, 4.6 nM, 1.5 nM, 0.5 nM.
[0113] 1.2 Reagents and instruments
[0114] Reagents: ALK5, purchased from Carna Company, Cat.No.09-141; p38α was purchased from Carna Company, Cat.No.04-152; TGFβR1 peptide was purchased from SignalChem Company, Cat.No.T36-58; II Methyl sulfoxide (DMSO), purchased from Sigma, USA; EDTA, purchased from Sigma, USA; ADP-Glo Kinase Assay, purchased from Promega, Cat.No.v9102 / 3, 1×kinase buffer ( 40mM Tris, pH 7.5, 0.10% BSA, 20mM MgCl 2 , 1mM DTT), prepared just before use.
[0115] Instrument: 2104Multilabel Reader, purchased from Perkin Elmer, USA.
[0116] 2. Experimental method
[0117] 2.1 Prepare 1x Kinase Buffer
[0118] 1x as...
experiment example 2
[0151] Experimental example 2 compound in vitro cell luciferase test evaluation
[0152] 1. Experimental materials
[0153] Test compound: the compound of formula (I) of the present invention in Example 1, prepared into 4mM with DMSO, and then diluted 4 times successively to 20000.00nM, 5000.00nM, 1250.00nM, 312.5nM, 78.125nM, 19.53nM, 4.88nM, 1.22 nM.
[0154] Luc-Smad2 / 3-NIH3T3 mouse fibroblasts (engineered to overexpress SMAD2, 3-responsive promoter) were donated by the laboratory of China Pharmaceutical University.
[0155] Reagents: DMEM, purchased from Invitrogen, USA; FBS, purchased from Invitrogen, USA; DMSO, purchased from Sigma, USA; Glo Lysis Buffer, purchased from Progema, USA; Bright-GloLuciferase assay system, purchased from USA Promega; TGFβ, purchased from PeproTech, USA.
[0156] Instrument: MD SpectraMax M3 multifunctional microplate reader, purchased from Molecular Devices, USA.
[0157] 2. Experimental method
[0158] 2.1 Cell culture:
[0159] Cell r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com