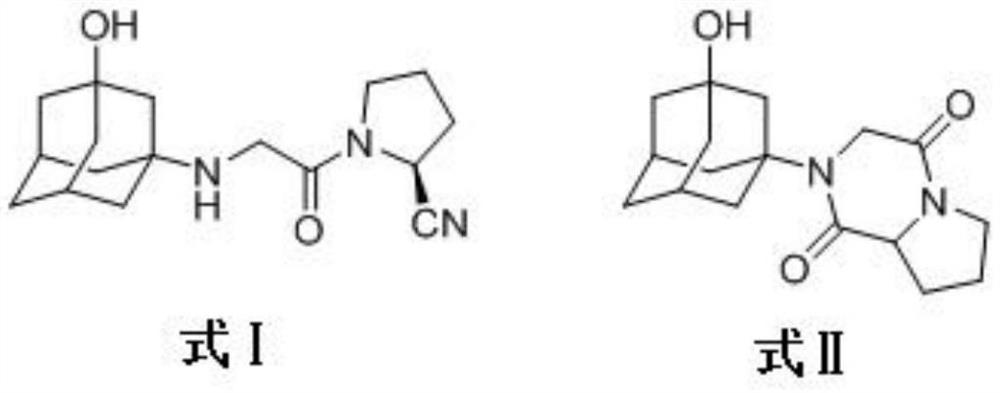
Vildagliptin diketopiperazine and preparation method thereof
A technology for vildagliptin diketopiperazine and diketopiperazine, which is applied in the field of medicinal chemistry, can solve the problems of many reaction steps, many side reactions, complicated processes and the like, achieves mild and easy controllable reaction conditions, reduced synthesis cost, The effect of the simple method of synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
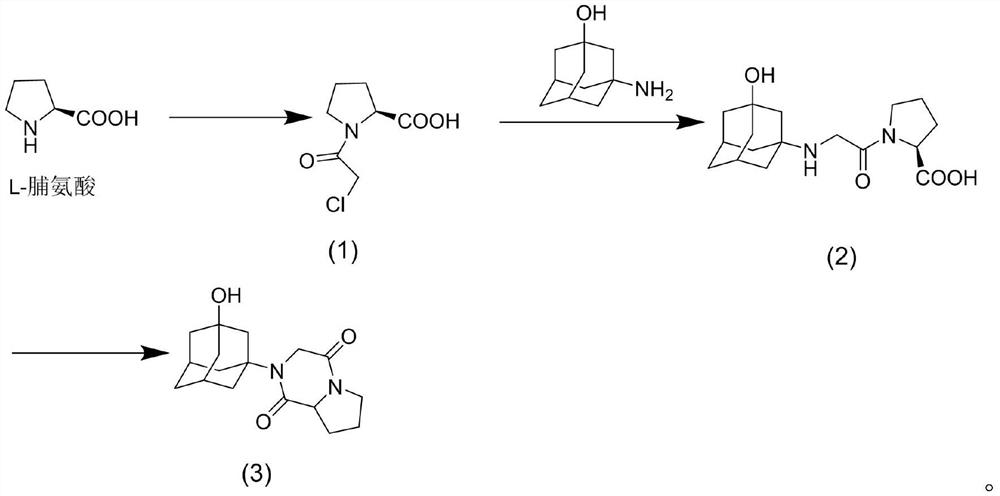
[0029] A preparation method of vildagliptin diketopiperazine, comprising the following steps:
[0030] S1, the synthesis of compound 1
[0031] Add 5g (0.044mol) of L-proline and 50ml (0.62mol) of tetrahydrofuran into a 150ml three-necked flask and stir, slowly add 5.04ml (0.067mol) of chloroacetyl chloride dropwise under ice-bath conditions, and heat to 40 Reflux at ℃ for 2h, cool, add 100ml of saturated sodium bicarbonate to wash; then add 100ml of ethyl acetate for extraction, the aqueous layer is extracted with ethyl acetate (20ml×3), the combined organic layers are dried with anhydrous sodium sulfate for 10h, and concentrated under reduced pressure A light yellow oil was obtained, which was recrystallized by slowly adding isopropyl ether dropwise in an ice bath, filtered, and the filter cake was dried to obtain 7.34 g of a white solid, with a yield of 87.5%, mp: 106-108°C;
[0032] S2, the synthesis of compound 2
[0033] 4.79g (0.025mol) of compound 1 was added in 20ml...
Embodiment 2
[0037] A preparation method of vildagliptin diketopiperazine, comprising the following steps:
[0038] S1, the synthesis of compound 1
[0039] Add 5g (0.044mol) of L-proline and 50ml of ethyl acetate into a 150ml three-neck flask and stir, slowly add 5.04ml (0.067mol) of chloroacetyl chloride dropwise under ice-bath conditions, and heat to reflux at 40°C after dropping 2h, cooled, added 100ml of saturated sodium bicarbonate for washing; then added 100ml of ethyl acetate for extraction, the aqueous layer was extracted with ethyl acetate (20ml×3), the combined organic layers were dried over anhydrous sodium sulfate for 10h, concentrated under reduced pressure to obtain Yellow oil, recrystallized by slowly adding isopropyl ether dropwise in ice bath, filtered, and dried the filter cake to obtain 7.50 g of white solid, yield 89.0%, mp: 106.5~107.5°C;
[0040] S2, the synthesis of compound 2
[0041]4.5g (0.023mol) of compound 1 was added in 20ml of acetonitrile to make a soluti...
Embodiment 3
[0045] A preparation method of vildagliptin diketopiperazine, comprising the following steps:
[0046] S1, the synthesis of compound 1
[0047] Add 10g (0.087mol) of L-proline and 100ml of tetrahydrofuran into a 250ml three-neck flask and stir, slowly add 8.5ml (0.114mol) of chloroacetyl chloride dropwise under ice-bath conditions, and heat to reflux at 40°C for 2h after dropping. Cool, add 200ml of saturated sodium bicarbonate to wash; then add 200ml of ethyl acetate to extract, the aqueous layer is extracted with ethyl acetate (20ml×3), the combined organic layer is dried with anhydrous sodium sulfate for 10h, and concentrated under reduced pressure to obtain a light yellow oil Recrystallized by slowly adding isopropyl ether dropwise in an ice bath, filtered, and dried the filter cake to obtain 15.20 g of white solid, yield 91.20%, mp: 106-108°C;
[0048] S2, the synthesis of compound 2
[0049] Add 3g (0.015mol) of compound 1 to 20ml of dimethylformamide to prepare a solu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com