Application of mesenchymal stem cell apoptotic body to preparation of medicine for treating bone defects
A technology of mesenchymal stem cells and apoptotic bodies, applied in medicine, in the field of mesenchymal stem cell apoptotic bodies, can solve the problems that cannot fundamentally solve the problems of damaged bone cell repair and bone tissue regeneration, and achieve quality Stabilizes, promotes bone regeneration, and promotes the effect of new bone formation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Induction of bone marrow mesenchymal stem cell apoptosis in vitro
[0044] Bone marrow mesenchymal stem cells cultured to the third generation 6 Inoculate to a 10cm culture dish at a density of one / dish and place at 37°C, 5% CO 2 The culture was continued under the conditions, and when the cell confluency reached 80%-85%, a dimethyl sulfoxide solution of staurosporine was added for induction, and the induction time was 4 hours.
[0045] Among them, the culture medium for the subculture of mesenchymal stem cells includes 500 parts by volume of α-MEM basal medium, 55.5 parts by volume of fetal bovine serum, penicillin with a concentration of 100 U / mL, and streptaline with a concentration of 100 U / mL. Mycin.
[0046] The solvent of staurosporine solution is dimethyl sulfoxide, 5mg staurosporine is dissolved in the dimethyl sulfoxide solution of 2.1435mL, the concentration that prepares is 5mm stock solution, staurosporine is in culture medium The working conc...
Embodiment 2
[0047] Example 2: Preparation of apoptotic bodies of mesenchymal stem cells
[0048] The supernatant from the induced apoptotic cells of Example 1 was collected in a sterile centrifuge tube (about 10ml in a 50ml tube), and the supernatant was centrifuged at 4°C and 800×g for 10min, and transferred to the Supernatant, remove cell debris; then centrifuge at 4°C, 16,000×g for 30 min, discard supernatant, collect precipitate, and resuspend in PBS; in order to remove the effect of reagents or serum in the medium, resuspend at 4 Centrifuge again at 16000×g for 30 min to obtain apoptotic bodies with higher purity as precipitates.
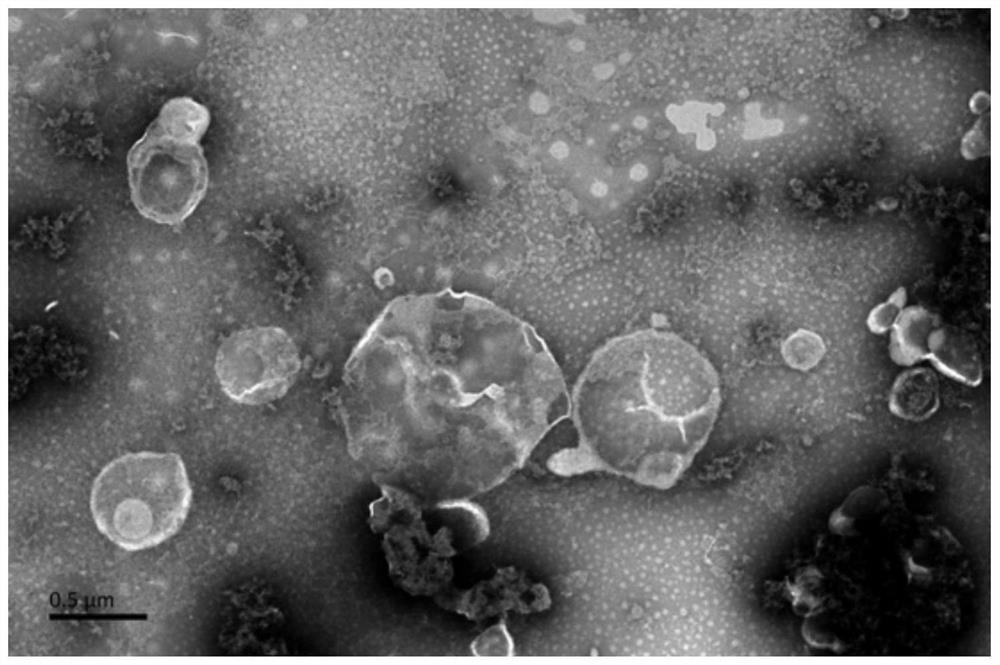
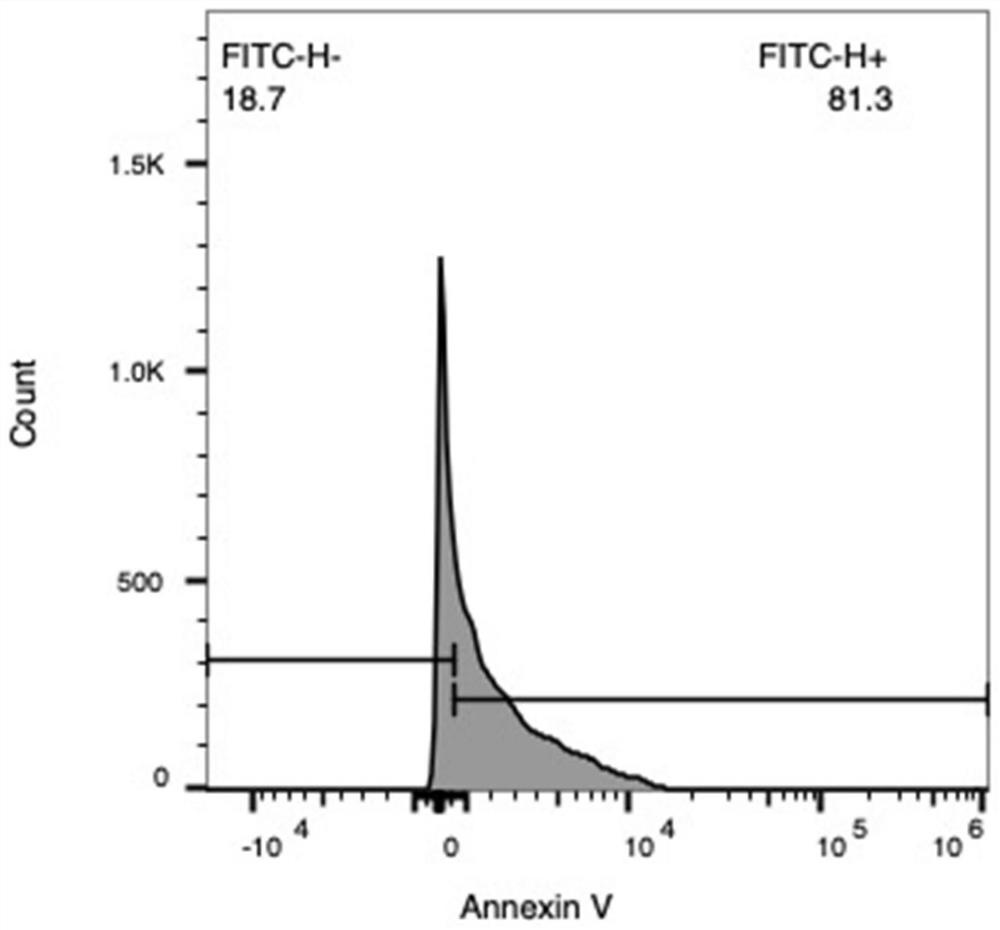
[0049] The diameter of the precipitated apoptotic body is 1-5 μm. The precipitated apoptotic bodies were then resuspended in 200 μL-400 μL PBS, aliquoted into sterile centrifuge tubes, and stored at -80°C for later use. At the same time, the basic morphology and markers of the apoptotic bodies were identified. The results are shown in Figure 1, Figure 1...
Embodiment 3
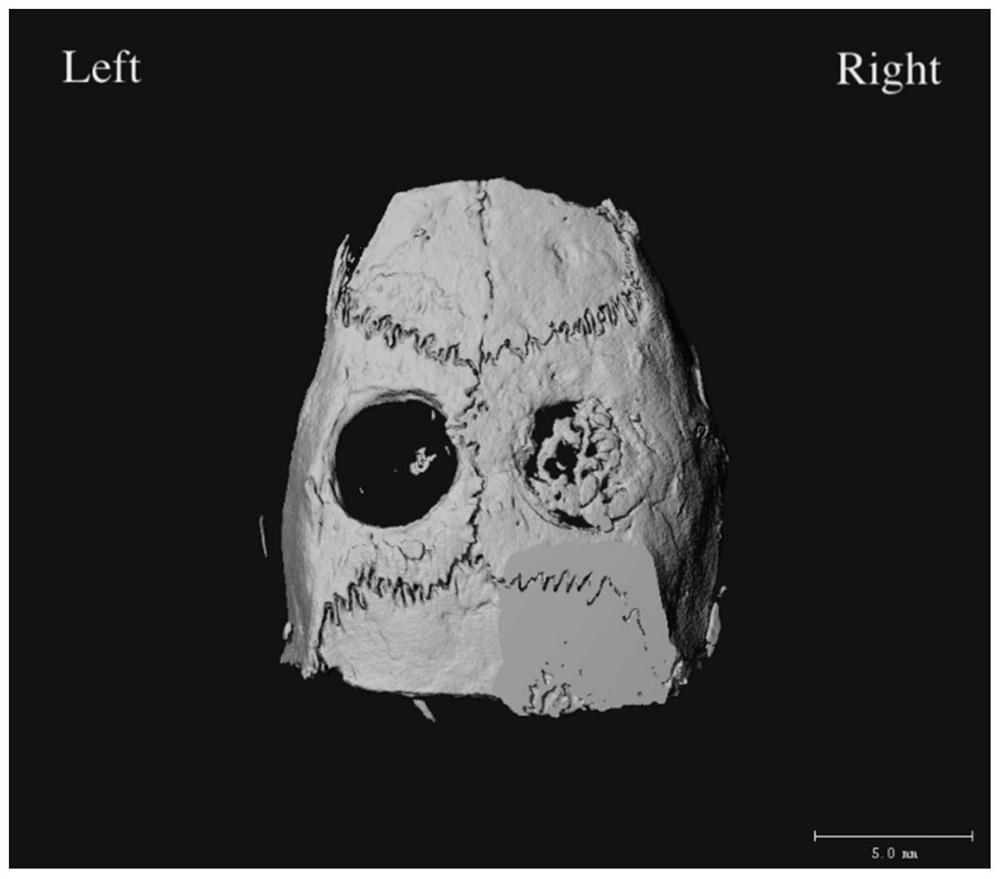
[0050] Example 3: Establishment, treatment and microCT detection of SD rat skull defect model
[0051] Select SD female rats about 4 weeks old, carry out intraperitoneal injection anesthesia with 1% sodium pentobarbital (40mg / kg), fix on the experimental table in prone position, connect the animal artificial respirator, the respiratory rate is about 86 times / min, The tidal volume was 15ml, and the respiratory ratio was set at 1:1. The head was depilated and skinned, and a drape was spread with disinfection. The skin was incised at the center of the skull at about the cranial suture, and the subcutaneous tissue was bluntly separated to visualize the skull. Connect the grinding machine, and cut the skull with a 5mm trephine at a speed of 1500r.p.m. or lower, so that the circular skull piece with a diameter of 5mm can be completely removed. During the operation, 1 drop of sterile saline was instilled every 2 seconds to flush the trephine and the skull (slow flushing speed is the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com