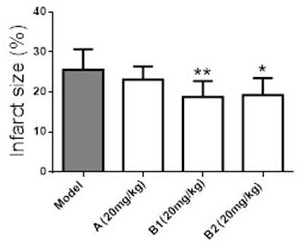
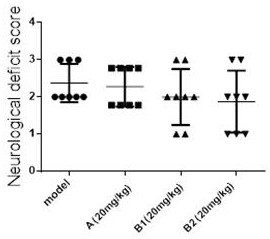
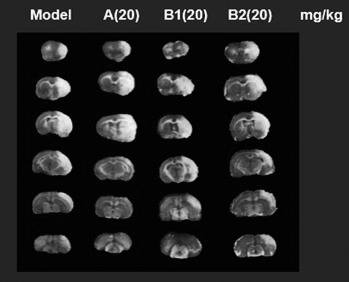
Leonurine borneol derivative as well as preparation method and application thereof
A technology of motherwort and derivatives, applied in the field of medicine, can solve problems such as unbeneficial motherwort borneol derivatives, etc., and achieve the effects of repairing nerve cell damage and improving survival rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1: The synthetic route of M-1 is as follows:
[0057]Add camphene (15.0g, 0.1mol), 2-bromoethanol (13.6g, 0.11mol) and montmorillonite K-10 (30.0g) into dichloromethane (200mL), stir at room temperature for 24 hours, and the reaction is complete After filtering, the filter cake was washed with saturated brine (100 mL), dried over anhydrous sodium sulfate, concentrated under reduced pressure, and column chromatography gave colorless oil M1 with a yield of 72%; ESI-MS: (m / z, %)= 260 [M+H] + .
Embodiment 2
[0058] Embodiment 2: The synthetic route of M-2 is as follows:
[0059] Dissolve syringic acid (5.94g, 30mmol) in 30mL of dichloromethane to obtain a dichloromethane solution of syringic acid, add potassium hydroxide (3.0g, 54mmol), potassium carbonate (4.1g, 30mmol), tetrabutyl ammonium bromide (1.0g, 3 mmol) and water 30mL, slowly add M-1 (7.8g, 30mmol) in dichloromethane (10mL) solution dropwise under stirring, dropwise in 30 minutes, react at room temperature for 24 hours, After the reaction was complete, the pH was adjusted to 5, the organic layer was separated, and the organic phase was washed with water in an amount of 30 mL. After washing with water, it was concentrated to dryness. M-2 was obtained by column chromatography with a yield of 51%. 1 H NMR (500 MHz, DMSO- d 6 ) δ = 7.28 (s, 2H), 4.13(t, 2H), 3.81 (s, 6H), 3.76 (t, 2H), 3.04 (m, 1H), 1.86-1.55 (m, 7H), 1.01(s , 3H), 0.97 (s, 6H). ESI-MS: (m / z, %) = 379 [M+H] + .
Embodiment 3
[0060] Embodiment 3: The synthetic route of M-4 is as follows:
[0061] M-2 (6.3 g, 16.66 mmol) was dissolved in 100 mL of DCM at room temperature, M-3 (6 g, 24.98 mmol), 4-dimethylaminopyridine p-toluenesulfonate (9.8 g, 33.32 mmol), N, N'-diisopropylcarbodiimide (4.2 g, 33.32 mmol), reacted for 5 h, washed with water, extracted with DCM, collected the lower layer solution, evaporated to dryness, and obtained white foamy solid by column chromatography M-4, yield 67%.
[0062] 1 H NMR (500 MHz, DMSO- d 6 ) δ = 9.31 (s, 1H), 8.33 (s, 1H), 7.20 (s,2H), 4.24-4.20 (m, 1H), 4.13 (t, 2H), 4.02 (q, 1H), 3.80 (s , 6H), 3.75 (t,2H), 3.04 (m, 1H), 1.98-1.56 (m, 13H), 1.46 (s, 9H), 1.37 (s, 9H), 1.01 (s,3H), 0.97 ( s, 6H). ESI-MS: (m / z, %) = 692 [M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com