PH-responsive T1/T2 switching type MRI contrast agent as well as preparation method and application thereof
A contrast agent, switchable technology, applied in products, T1/T2 switchable MRI contrast agent and its preparation field, can solve the problem that the switchable contrast agent is rarely reported, and achieve enhanced high permeability and retention effect, improve imaging contrast, good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0046] Another aspect of the embodiment of the present invention also provides a pH response T 1 / T 2 Preparation method of switching MRI contrast agent, including:
[0047] A ring-containing polymerized polymerized reaction is carried out at least a first homogenate ring-containing mixed reaction system containing glutamate and propynamine to prepare a group-containing poly γ-benzyl-L-glutamic acid;
[0048] At least a reaction comprising the alkynyl group-containing poly γ-benzyl-L-glutamic acid is reacted with a second homogeneous mixed reaction system of the brominated acetic acetic acid solution to prepare a group-containing poly-L-glutamic acid;
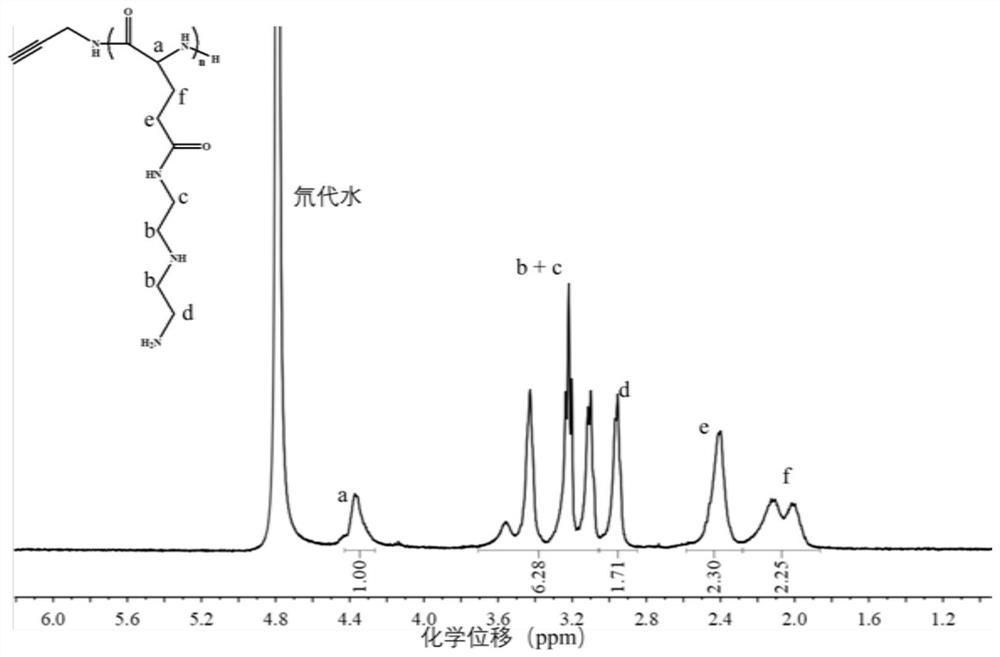
[0049] At least the third uniform mixed reaction system containing the alkynyl group with 2-hydroxypyridine, a tertiary mixed reaction system of 2-hydroxypyridine, and a divinity, and is prepared to prepare a polyol-containing gauge {n- [N '- (2-aminoethyl) -2-aminoethyl] glutamate};
[0050] At least the polythynyl {N- [N- [N- (2...
Embodiment 1
[0104] A pH response T 1 / T 2 The preparation method of the switching MRI contrast agent (ESIONPS system) includes the following steps:
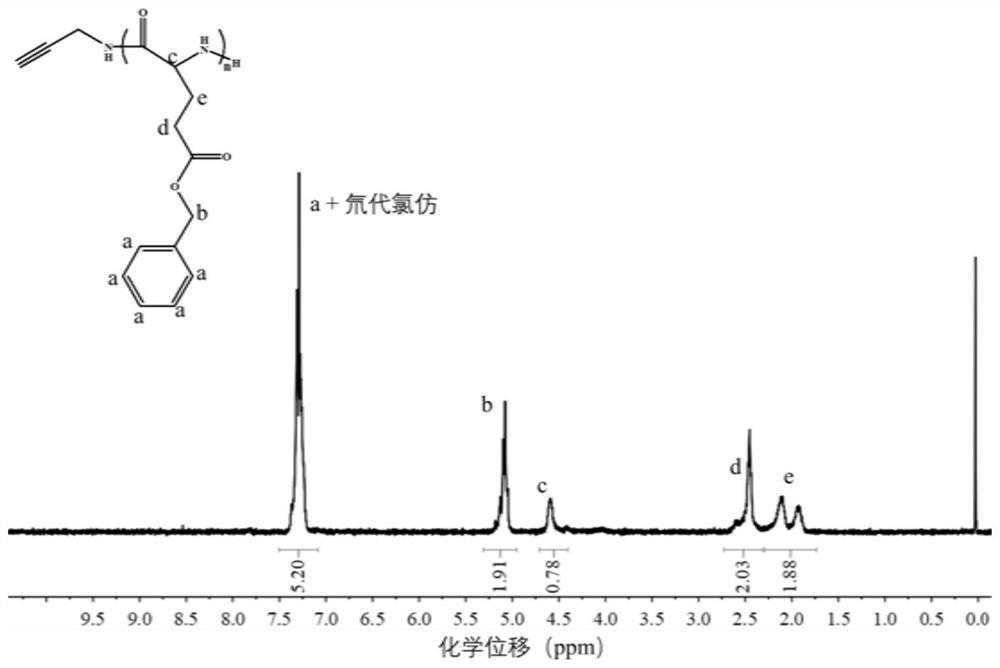
[0105] (1) Preparation of the allergy-containing poly γ-benzyl-L-glutamic acid: dichloromethane is stirred overnight by calcium hydride overnight and distilled to obtain a dry solution, dimethylformamide is dried over hydrogenated calcium and reduced pressure distillation The dried solution was obtained, and the glutamate chloride (BLG-NCA) was dissolved in a protective atmosphere in dichloromethane and dimethylformamide (volume ratio of 4: 1), and propargymine was added. 25 ° C reaction 24 h, where the molar ratio of the glutamic acid cyclic anhydride and the propargylamine is 40: 1, and the obtained solution is slowly added dropwise into the ice ether after the reaction is completed, and the solid is collected, dissolved in chlorine. EtOAc EtOAc EtOAc EtOAc,
[0106] (2) Preparation of the alkynyl group-containing poly-L-glutamic acid: alkyny...
Embodiment 2
[0119] A pH response T 1 / T 2 Preparation method of switching MRI contrast agent, including the following steps:
[0120] (1) Step (1) in Example 1, only the reaction time of glutamate chromataric anhydride (BLG-NCA) and the propargylamine were adjusted to 22 ° C for 36 h, and glutate benzyl ester The molar ratio of the rheinated acid anhydride and propargylamine is 50: 1;
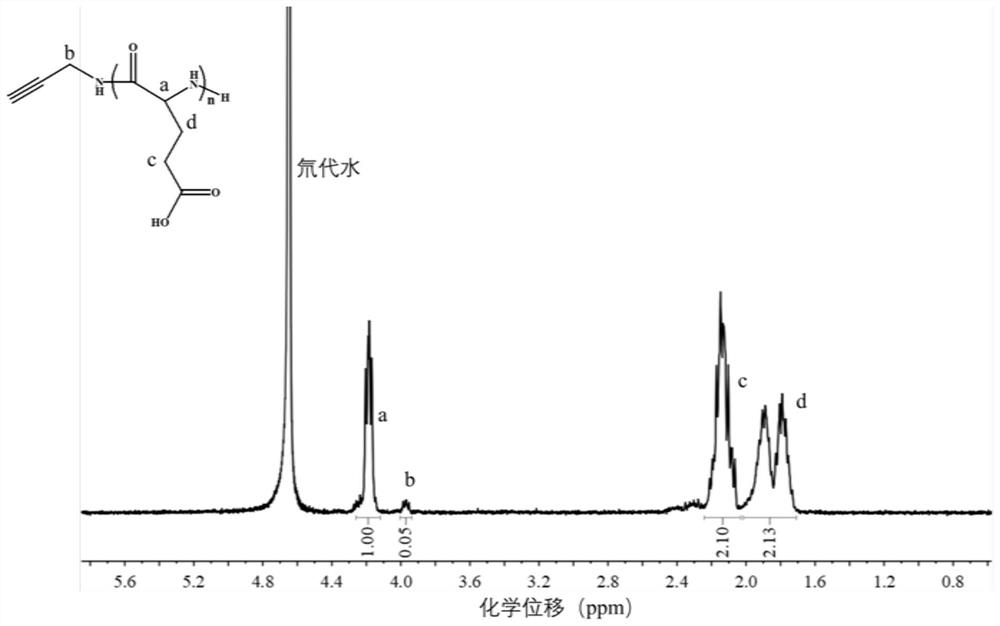
[0121] (2) This step is similar to the step (2) in Example 1, and the alkynyl-containing poly γ-benzyl-glutamic acid is dissolved in trifluoroacetic acid trifluoroacetic acid in trifluoroacetate. In a 100 mL round bottom flask, a 33 wt% bromide solution was added, and then at 55 ° C for 3 h, it was reduced to a room temperature reaction for 18 h, resulting in a group-containing poly-L-glutamic acid;
[0122] (3) This step is similar to the step (3) in Example 1, only the alkynyl group-containing poly γ-benzyl-L-glutamic acid with 2-hydroxypyridine molar ratio of benzyl and 2-hydroxypyridine Adjusting to 1: 8,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Longitudinal relaxation rate | aaaaa | aaaaa |
| Transverse relaxation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com