Method for capturing SO2 through PCET reaction electrochemical cycling
A kind of SO2, electrochemical technology, applied in the field of desulfurization, can solve the problems of high energy consumption, low cost, poor stability, etc., and achieve the effect of alleviating the pressure of scarcity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
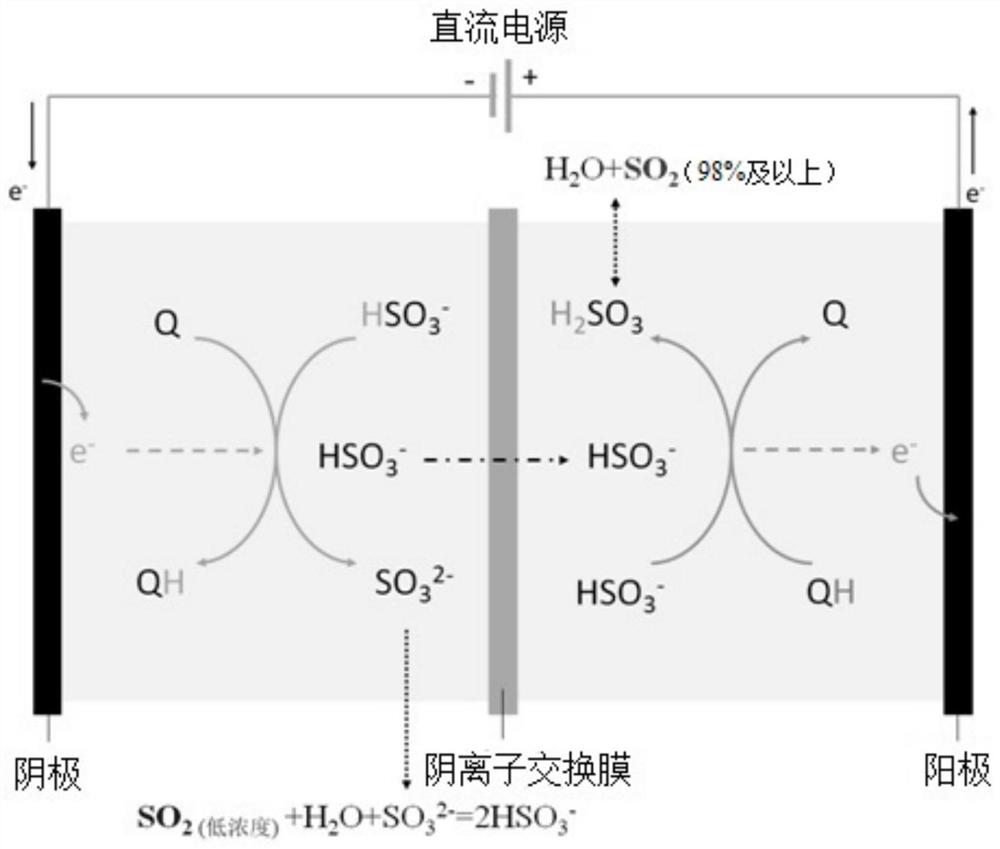
[0045] Embodiment one, see figure 1 , the electrolytic cell is divided into an anode area and a cathode area by an anion exchange membrane, and the anode electrode and the cathode electrode are made of carbon fiber cloth.
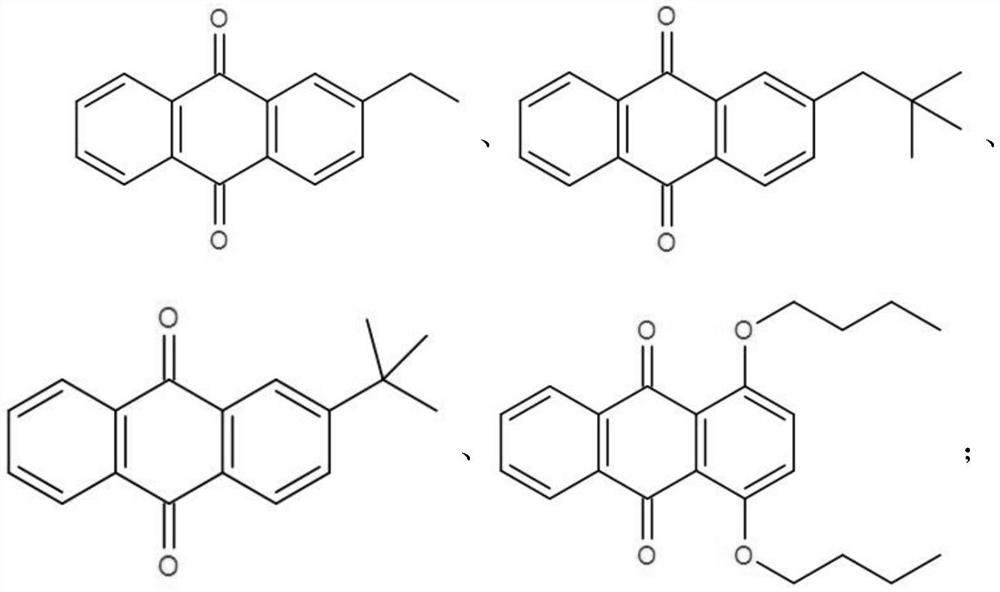
[0046] use As the cathode electrocatalyst Q, the as the anode electrocatalyst QH.
[0047] Compound Q and compound QH were dissolved in 1,2 dichloroethane respectively, and the 1-butyl-3-methylimidazolium hexafluorosulfate ionic liquid with a concentration of 0.3mol / L was added as a supporting electrolyte, and the compound The concentrations of Q and compound QH in 1,2 dichloroethane are both 0.2mol / L. Configure 20%wt NaHSO respectively 3 solution and 20%wt Na 2 SO 3 solution. Will SO 2 into Na 2 SO 3 React in solution to form NaHSO 3 , so that the solution also contains HSO 3 - , SO 3 2- , and adjust the pH of the solution to 6-7. NaHSO 3 The solution and the 1,2-dichloroethane solution dissolved with compound QH are mixed and passed int...
Embodiment 2
[0059]Embodiment two, see figure 2 , the electrolytic cell is divided into an anode area and a cathode area by using a cation exchange membrane, and both the anode electrode and the cathode electrode are made of carbon fiber paper.
[0060] use As the cathode electrocatalyst Q,
[0061] use as the anode electrocatalyst QH.
[0062] Dissolve compound Q and compound QH in chloroform respectively, and add the 1-butyl-3-methylimidazolium hexafluorosulfate ionic liquid that concentration is 0.3mol / L as supporting electrolyte, described compound Q and compound QH The concentration in chloroform is 0.1mol / L. 30%wt NH were configured separately 4 HSO 3 solution and 30%wt (NH 4 ) 2 SO 3 solution, the low concentration SO 2 Respectively passed into the ammonium sulfite solution to react to generate NH 4 HSO 3 , so that the solution also contains HSO 3 - , SO 3 2- , and adjust the pH of the solution to 5-7. Will NH 4 HSO 3 The solution and the chloroform solution di...
Embodiment 3
[0065] Embodiment 3, the electrolytic cell is divided into an anode area and a cathode area by using a cation exchange membrane, and both the anode electrode and the cathode electrode are made of carbon fiber paper.
[0066] use As the cathode electrocatalyst Q, the as the anode electrocatalyst QH. Other process is identical with embodiment two.
[0067] In this example, the SO in the flue gas before capture 2 The concentration is 1550mg / m 3 , SO in flue gas after capture 2 The concentration is 48mg / m 3 , the set of SO 2 The concentration is greater than 99%. During electrolysis, the electrolysis current density is 10mA / cm -2 , the average electrolysis voltage is only 0.6V, the electrolysis efficiency is 75%, and every ton of SO captured 2 The energy consumption is only about 670kWh, and the cost is about 335 yuan per ton.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com