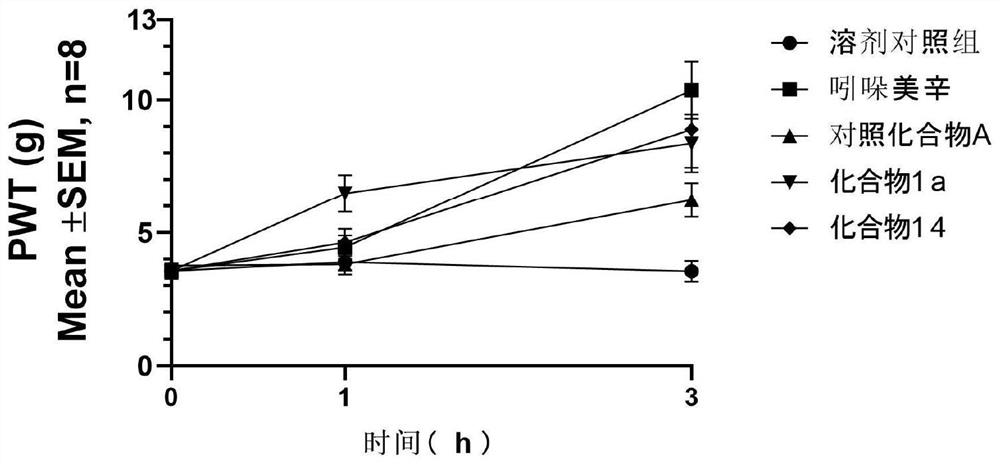
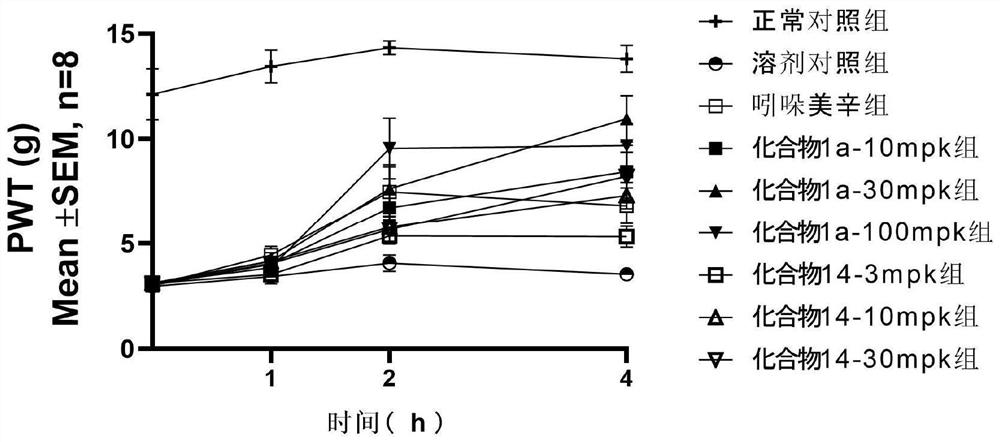
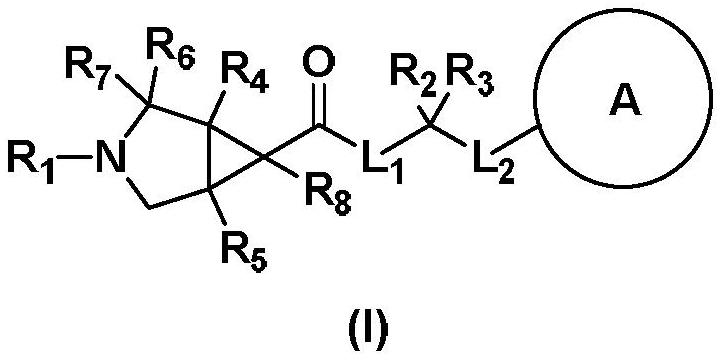
Fused ring compound and preparation method, pharmaceutical composition and application thereof
A technology of compounds and oxides, applied in drug combinations, antipyretics, organic chemistry, etc., can solve the problems of high toxicity and side effects, poor long-term safety, and low tolerance, and achieve high metabolic stability and excellent drug efficacy pharmacokinetic effect, excellent pharmacokinetic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0302] Embodiments of the present invention also provide a preparation method of the compound, comprising the steps of:
[0303]
[0304] Condensation reaction is carried out with compound 1 and compound 2, wherein Q represents a nitrogen protecting group;
[0305] The nitrogen protecting group Q is removed from the product of the condensation reaction.
[0306] Embodiments of the present invention also provide a pharmaceutical composition containing a therapeutically effective dose of the above-mentioned compound or its stereoisomer, N-oxide, hydrate, solvate, metabolite, pharmaceutical Pharmaceutically acceptable salts, polymorphs or prodrugs, and pharmaceutically acceptable carriers or excipients.
[0307] Embodiments of the present invention also provide a compound as described above or its stereoisomer, N-oxide, hydrate, solvate, metabolite, pharmaceutically acceptable salt, polymorph or prodrug, or as described above The above pharmaceutical composition is used in t...
Embodiment 1
[0357] (1R,5S,6r)-N-(2-(8-(Methylthio)imidazo[1,5-a]pyridin-3-yl)propan-2-yl)-3-azabicyclo[3.1 .0] hexane-6-carboxamide hydrochloride (compound 1)
[0358]
[0359] The first step: 2-cyano-3-methylthiopyridine (1B)
[0360]
[0361] 2-cyano-3-fluoropyridine (2.0 g, 16.38 mol) and sodium methylthiolate (1.2 g, 18.02 mmol) were added to dimethyl sulfoxide (10 mL), and the mixture was stirred at room temperature for 5 hours. Add ethyl acetate (120mL), wash with water and saturated brine, spin the organic phase to dry the solvent in vacuo, and purify the residue on a silica gel column (petroleum ether / ethyl acetate=3 / 1) to obtain a white solid, which is 2-cyano - 3-Methylthiopyridine 1B (900 mg, yield: 36.6%).
[0362] The second step: (3-(methylthio)pyridin-2-yl)methanamine dihydrochloride (1C)
[0363]
[0364] Dissolve 2-cyano-3-methylthiopyridine 1B (800mg, 5.33mmol) in ethanol (20mL), add 10% palladium / carbon (520mg, 0.53mmol) and hydrochloric acid (6M, 5mL), and ...
Embodiment 2
[0385] (1R,5S,6r)-N-(2-Methyl-1-((2-(trifluoromethyl)phenyl)thio)prop-2-yl)-3-azabicyclo[3.1.0 ]Hexane-6-carboxamide formate (compound 2)
[0386]
[0387] The first step: 2-methyl-2-nitropropyl 4-methylbenzenesulfonate (2B)
[0388]
[0389] 2-Methyl-2-nitropropan-1-ol 2A (1.19g, 10.00mmol) and triethylamine (2.02g, 20.00mmol) were mixed and dissolved in dichloromethane (50mL), cooled to 0-5 ℃. Add 4-methylbenzenesulfonyl chloride (2.86 g, 15.00 mmol), and stir at room temperature for 18 hours. New spots were detected by TLC. Add dichloromethane (100 mL) to dilute, wash with 1M hydrochloric acid (100 mL) and saturated sodium bicarbonate solution (100 mL), and then wash with saturated brine. The organic phase was separated, dried with anhydrous sodium sulfate, filtered, and purified with a silica gel column (petroleum ether / ethyl acetate=10 / 1) to obtain 2-methyl-2-nitropropyl 4-methylbenzenesulfonate Ester 2B, off-white solid (2.46 g, yield: 90%).
[0390] 1 H NMR...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com