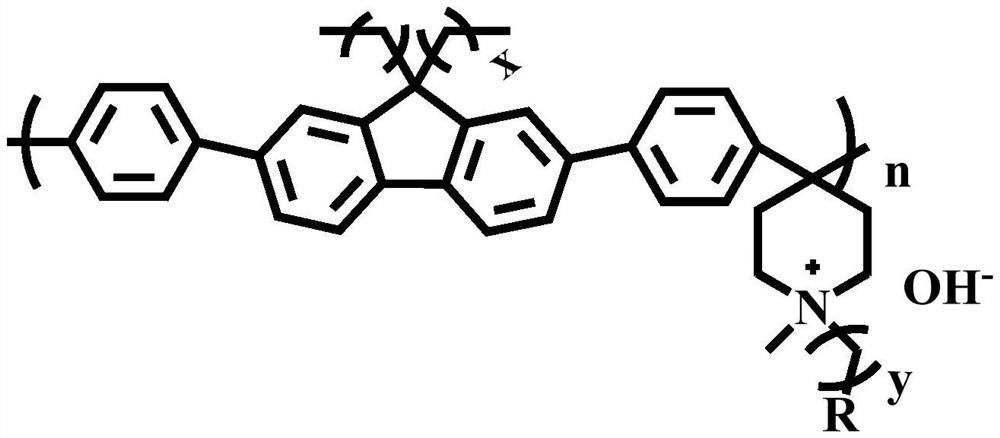
Main chain type alkaline anion exchange membrane based on ether-bond-free polyfluorene and preparation method thereof
An anion exchange membrane and basic anion technology, applied in chemical instruments and methods, membrane, membrane technology, etc., can solve the problems of few application reports in the field of fuel cells, achieve excellent chemical stability, increase molecular weight, and improve ion conductivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
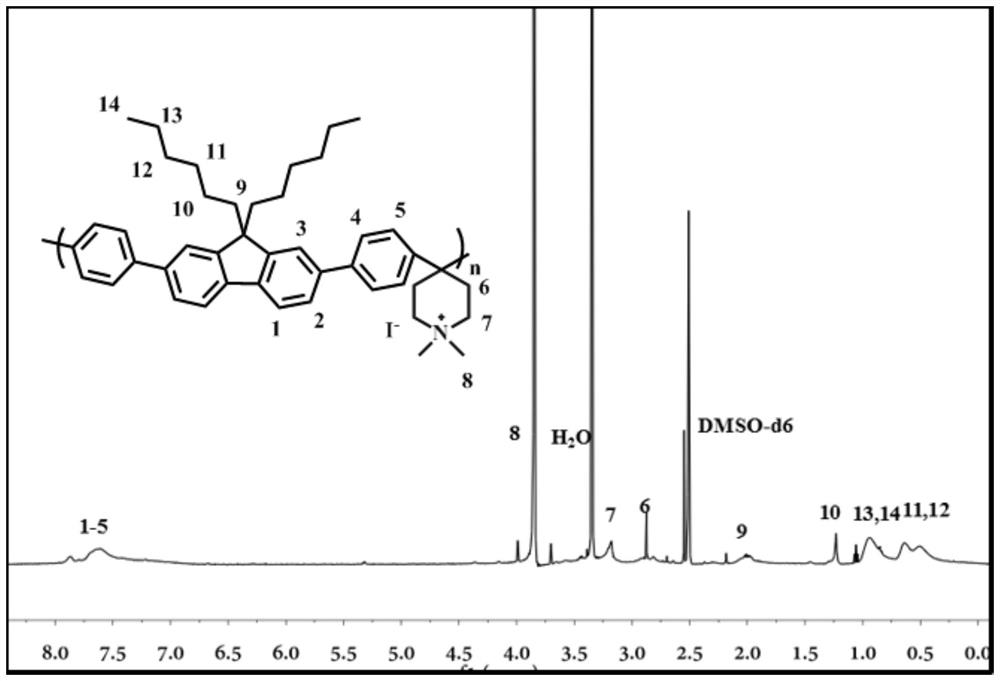
[0027] (1) Dissolve 9,9-dihexyl-2,7-dibromofluorene (1.72g, 3.5mmol) in 35ml of toluene, add 35ml of 1M Na 2 CO 3 aqueous solution. The mixture was heated to 100°C, and phenylboronic acid (1.28 g, 10.5 mmol) and Pd (pph 3 ) 4 (4.2mg, 0.1mmol), stirred for 36 hours. The reaction solution was extracted with dichloromethane and water, and the dried organic layer was collected. The crude product was separated and purified by column chromatography using a mixture of n-hexane and dichloromethane with volume gradient changes to obtain 9,9-dihexyl-2,7-diphenylfluorene with a yield of ~95%.
[0028] (2) Dissolve 9,9-dihexyl-2,7-diphenylfluorene (2.2g, 4.5mmol) and N-methyl-4-piperidone (0.56g, 4.95mmol) in 9ml of dichloromethane middle. Add 0.5ml of trifluoroacetic acid and 6ml of trifluoromethanesulfonic acid successively under ice-water bath. The mixture was mechanically stirred at room temperature for 48 hours, and the mixture became highly viscous. Na 2 CO 3 The aqueous s...
Embodiment 2
[0032] This example is similar to Example 1, except that n-butane is modified at the 9th position of fluorene, and its chemical structure is shown in the figure:
[0033] The prepared anion exchange membrane has an ion conductivity of 70.11mS cm at 80°C -1 , The degree of swelling at 80°C is only 6.1%. The tensile strength is 21.2MPa. When the film was soaked in 2M KOH solution at 80°C for 30 days, the conductivity lost only 12.42%.
Embodiment 3
[0035] This example is similar to Example 1, except that methane is modified at the 9th position of fluorene, and its chemical structural formula is shown in the figure:
[0036] The prepared anion exchange membrane has an ion conductivity of 66.68mS cm at 80°C -1 , The degree of swelling at 80°C is only 5.7%. The tensile strength is 20.2 MPa. When the film was soaked in 2M KOH solution at 80°C for 30 days, the conductivity lost only 14.48%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ionic conductivity | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
| Ionic conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com