Electrochemical energy storage polymer electrolyte and preparation method thereof
A polymer and electrolyte technology, which is applied in the field of lithium-ion batteries, can solve problems such as uneven effects of external conditions, and achieve the effects of improving flame retardancy, improving ion transmission efficiency, and improving current efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
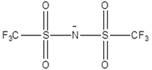
[0036] An electrochemical energy-storage polymer electrolyte and a preparation method. Ethylene carbonate and propylene glycol dipropionate are selected as polymer monomers (monomer ratio 1:1), and the monomers are dissolved in N-methylpyrrolidone at 60° C. medium; the electrolyte lithium salt LiFSI (1mol / L), ionic liquid EMImFSI (2mol / L, the structure is as follows), silicon dioxide (content 3%), dibenzamide peroxide (content 0.5%), citric acid (content 5%) was added to the aforementioned system, heated at 80 °C for 12 h; finally, the excess solvent was removed by rotary evaporation at 100 °C.
[0037] EMimFSI
[0038] The conductivity test method adopts the traditional film casting method, and then loads it into a button battery, and conducts the conductivity test with commercial lithium iron phosphate and lithium sheets as electrodes. The measured ionic conductivity at 30°C is 3.4×10 -3 .
Embodiment 2
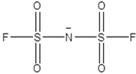
[0040] An electrochemical energy storage polymer electrolyte and a preparation method, selecting methyl ethyl carbonate and methyl methacrylate as polymer monomers (monomer ratio 1.5:1), and dissolving the monomers in N methyl at 60° C. In pyrrolidone; the electrolyte lithium salt LiPFSI (1mol / L), the ionic liquid MMimPFSI (1.5mol / L, the structure is as follows), titanium dioxide (content 3%), azobisisobutyronitrile (content 2.5%), EDTA (content 2 %) was added to the aforementioned system, heated at 80 °C for 10 h; finally, the excess solvent was removed by rotary evaporation at 100 °C.
[0041]
[0042] The conductivity test method adopts the traditional film casting method, and then loads it into a button battery, and conducts the conductivity test with commercial lithium iron phosphate and lithium sheets as electrodes. The measured ionic conductivity at 30°C is 2.6×10 -3 .
Embodiment 3
[0044] An electrochemical energy storage polymer electrolyte and a preparation method, wherein vinylidene fluoride and hexafluoropropylene are selected as polymer monomers (monomer ratio 1:1), and the monomers are dissolved in N-methylpyrrolidone at 80°C; The electrolyte lithium salt LiPFSI (2mol / L), ionic liquid PYR13TFSI (1.5mol / L, the structure is as follows), aluminum oxide (content 5%), dimethyl azobisisobutyrate (content 1.5%), tartaric acid (content 5%) was added to the aforementioned system, heated at 90 °C for 8 h; finally, the excess solvent was removed by rotary evaporation at 100 °C.
[0045]
[0046] The conductivity test method adopts the traditional film casting method, and then puts it into a button battery, and uses commercial lithium iron phosphate pole pieces and lithium pieces as electrodes for conductivity testing. The measured ionic conductivity at 30°C was 5.2×10 -3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com