Engineered platelets of nanogel internally loaded with chemotherapeutic drugs and externally carried with immune checkpoint inhibitors as well as preparation method and application of engineered platelets
A technology of immune checkpoint and nanogel, which is applied in the direction of nanotechnology, nanotechnology, nanomedicine, etc., can solve the problems of high cost, low drug loading of preparations, and inability to target delivery, etc., to achieve high loading efficiency and prolong survival Long-term, high-safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
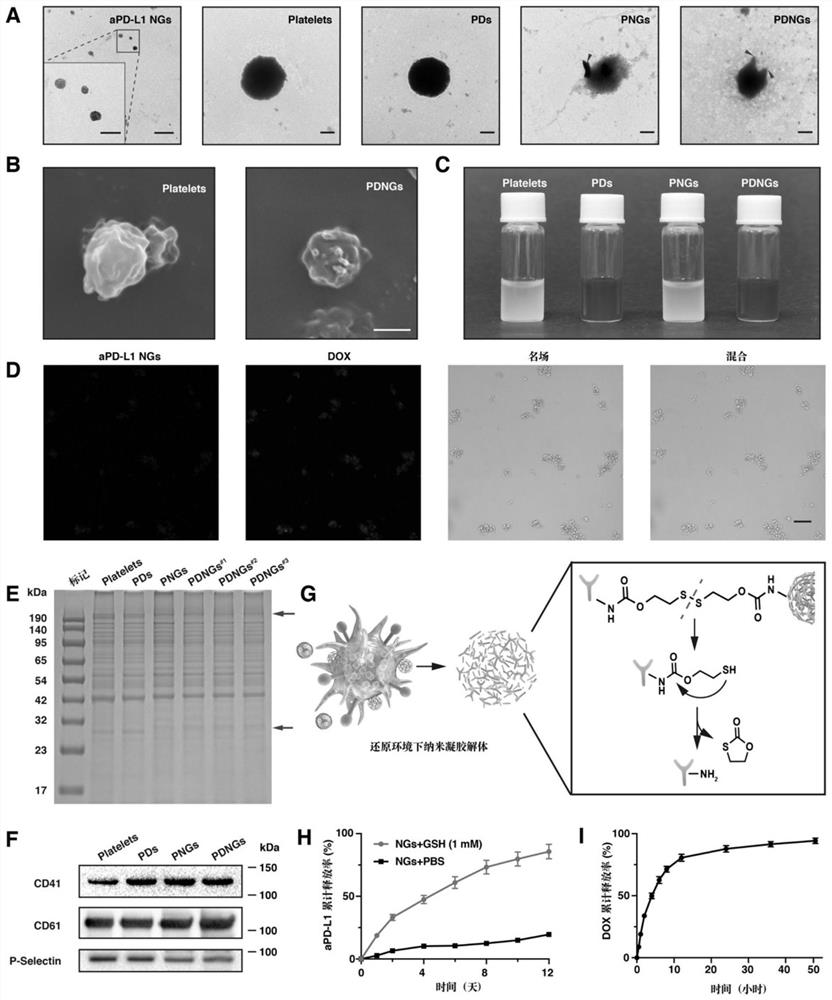
[0093] Example 1: Preparation method and characterization of aPD-L1 nanogel engineered platelets (PDNGs) loaded with doxorubicin inside and outside.
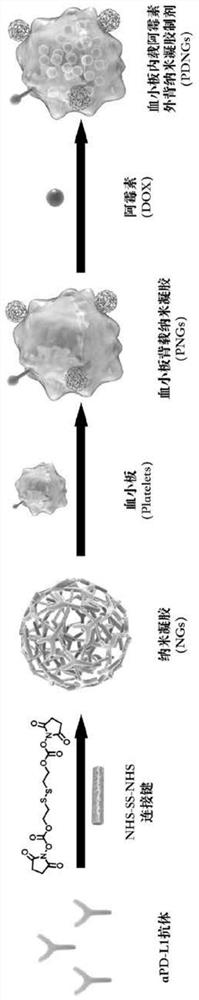
[0094] Synthesis schematic see figure 1 .
[0095] (1) aPD-L1 nanogels (NGs) as backpacks were prepared by mixing aPD-L1 antibody and NHS-SS-NHS cross-linking agent at a mass ratio of 1:15, stirring at room temperature for 1 hour, Filter the centrifuge tube (molecular weight cut-off 100kDa) and centrifuge at 4000rpm for 15 minutes, and wash with PBS three times to remove excess NHS-SS-NHS. The prepared aPD-L1 nanogel has a uniform particle size distribution of 100-130 nanometers.
[0096] (2) After the platelet-rich plasma was anticoagulated with EDTA, the red blood cells were removed by centrifugation at 200 g for 4 minutes, and the supernatant was taken and prostacyclin was added to inhibit platelet activation. Mix with an equal amount of ACD solution, centrifuge at 800g for 10 minutes, resuspend the platelets in Ren's solut...
Embodiment 2
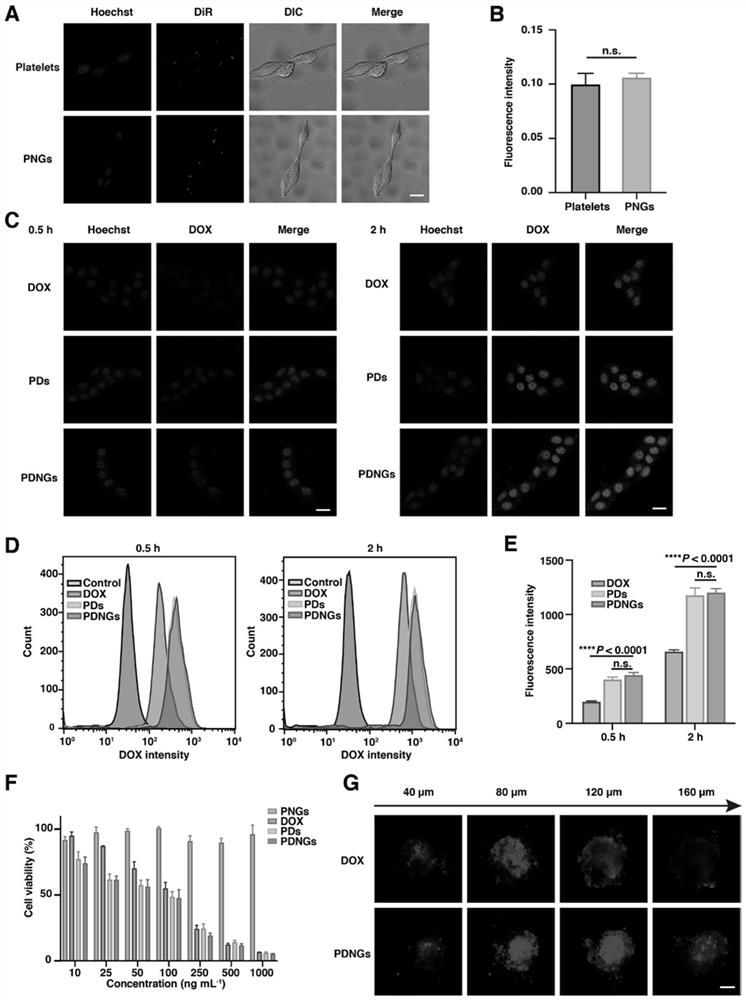
[0101]Example 2: Effect of PDNGs on B16-F10 cells in vitro.
[0102] First, the adhesion of PDNGs to B16-F10 cells was investigated. In order to avoid the interference of the fluorescence of doxorubicin itself, PNGs were used instead of PDNGs. DiR-labeled PNGs and DiR-labeled platelets were incubated with tumor cells for 1 hour, and confocal fluorescence microscopy showed that PNGs could adhere to the surface of tumor cells ( image 3 A), In addition, there is almost no difference in fluorescence intensity from attached platelets, further indicating that the outer back aPD-L1 nanogel has no significant effect on the physiological engineering of platelets ( image 3 B).
[0103] After incubation of B16-F10 cells with free DOX, PDs and PDNGs for 0.5 h and 2 h, confocal laser scanning microscopy (CLSM) ( image 3 C) Cellular uptake of doxorubicin was examined and quantified by flow cytometry ( image 3 D, 3E). Bioengineered platelets showed higher intracellular fluorescence i...
Embodiment 3
[0106] Example 3: To investigate the ability of PDNGs to target postoperative tumor sites in vivo.
[0107] In order to investigate the ability of PDNGs to target postoperative tumor sites in vivo, the tumors of B16-F10 tumor-bearing C57BL / 6 mice were incompletely excised, and DiR-labeled PDNGs and free DiR solution were injected through the tail vein immediately after surgery at different time points. In vivo imaging, such as Figure 4 As shown in A, DiR-labeled PDNGs showed stronger fluorescent signals at the tumor site and accumulated the most at the tumor site 6 hours after injection. The postoperative tumors and major organs were removed at 6 hours and 48 hours after injection, respectively, and fluorescence imaging showed that the kidneys and spleens of mice given DiR showed high fluorescence signals ( Figure 4 B). At 6 h and 48 h time points, the fluorescence intensity of the tumors administered with PDNGs was 6.78-fold and 3.10-fold higher than that of the tumors ad...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com