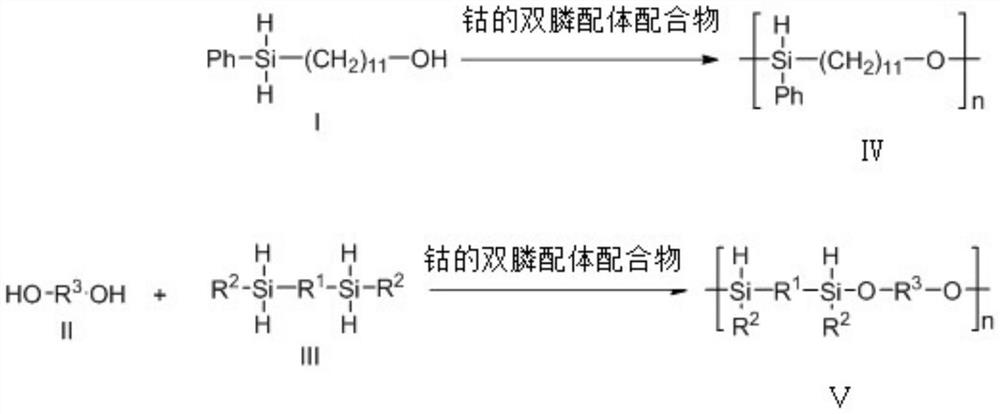
Polysilyl ether and method for synthesizing polysilyl ether through selective dehydrogenation coupling of prochiral silane and diol under catalysis of cobalt
A technology of dehydrogenation coupling and polysilicon ether, which is applied in the field of silicon-containing polymer synthesis, can solve the problems of undeveloped, unfavorable property adjustment of polysilicon ether, etc., and achieves simple and practical reaction operation, novel structure and convenient preparation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Synthesis of monomer 3a
[0050]
[0051] 1,6-bis(phenylsilyl)hexyl Alkane (3a). Under nitrogen protection, add Co(acac) in the reaction flask 2 (15.6 mg, 0.06 mmol), 4,5-bis(diphenylphosphine)-9,9-dimethylxanthene xantphos (34.8 mg, 0.06 mmol) and THF (10 mL). The solution was stirred at room temperature for 10 minutes. Then, phenylsilane 1 (4.544 g, 42 mmol) and 1,5-hexadiene (1.574 g, 19 mmol) were added to the bottle under nitrogen. The reaction vial was stirred at room temperature for 12 hours. Then, the resulting solution was concentrated in vacuo. The crude product was dissolved in petroleum ether, and the solution was quickly filtered through a short column of silica gel. The filtrate was collected and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (petroleum ether) to obtain a colorless oily liquid. In order to improve the polymerization performance of the monomer, a dehydration process must be perfo...
Embodiment 2
[0053] Synthesis of Monomer 6
[0054]
[0055] 11-(phenylsilyl)undecane- 14,5-Bis(diphenylphosphine)-9,9-dimethylxanthene-ol (6). Under nitrogen protection, add Co(acac) in the reaction flask 2 (7.7 mg, 0.03 mmol), xantphos (17.4 mg, 0.03 mmol) and THF (5 mL). The solution was stirred at room temperature for 10 minutes. Then, phenylsilane 1 (0.893 g, 8.3 mmol) and methyl 10-undecenoate 4 (1.486 g, 7.5 mmol) were added to the bottle under nitrogen. The reaction vial was stirred at room temperature for 24 hours. Then, the resulting solution was concentrated in vacuo. The crude product was dissolved in petroleum ether, and the solution was quickly filtered through a short column of silica gel. The filtrate was collected and concentrated under reduced pressure. Et was added to the crude product 2 O (30 mL). The mixture was cooled on an ice bath, and LiAlH was added carefully 4 (300 mg, 7.5 mmol). The reaction mixture was allowed to warm to room temperature and stirr...
Embodiment 3-18
[0057]Optimization of Dehydrocoupling Polymerization Conditions
[0058] In the glove box, put Co(acac) in the reaction bottle 2 (1 mol% of the amount of substrate used) and bisphosphine ligand (1 mol% of the amount of substrate used), tetrahydrofuran (1.0 mL) was added, stirred at room temperature for 5 min, and the tetrahydrofuran was dried under vacuum. Silane 3a (0.4mmol), diol 7a (0.4mmol) and reaction solvent (2.0mL) were added in a reaction vial. React at 60–80°C for 24 hours; then add 2 ml of dichloromethane to dissolve the product, add dropwise 15 ml of cold methanol to precipitate the product, remove the upper solvent, and drain to obtain the polymer product. The reaction formula and ligand structure are as follows:
[0059]
[0060] The number average molecular weight of the polymer (M n ) and molecular weight distribution (PDI) are measured by gel chromatography (GPC), and the productive rate is the separation yield, changing the reaction temperature and solve...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com