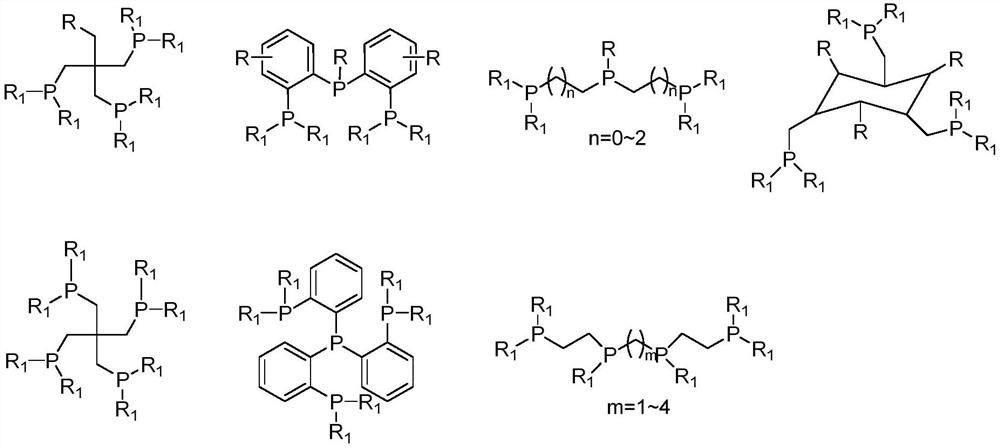
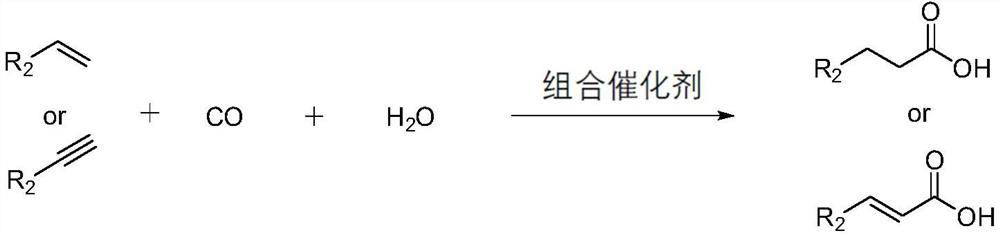
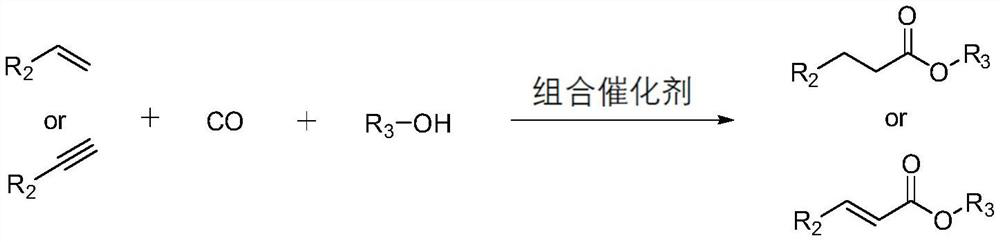
Method for preparing straight-chain carbonyl compound by catalyzing unsaturated hydrocarbon through polydentate phosphine ligand modified palladium combined catalyst
A technology combining catalysts and carbonyl compounds, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, preparation of organic compounds, etc., can solve problems such as few reports of straight-chain carbonyl compounds, and achieve the goal of using Effects of long lifetime, high chemical and regioselectivity, and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1--14
[0035] (1) The reaction results of different palladium compounds and phosphine ligands for the carbonylation and carboxylation of styrene to prepare phenylpropionic acid
[0036]Specific experimental steps: Add 0.01mmol of palladium compound, 0.012mmol of multidentate phosphine ligand, 0.05mmol of p-toluenesulfonic acid, 20mmol of styrene, 5mL of water and N-methylpyrrolidone into 200mL of polytetrafluoroethylene lining Solvent (NMP 40 mL). Put the liner in the autoclave, seal it and check the airtightness of the device, and replace the air in the autoclave with carbon monoxide. Then, carbon monoxide gas was introduced and the pressure was increased to 3.0 MPa, and the reaction was carried out in a constant temperature heating mantle at 120°C for 4 hours, and the pressure was released slowly after cooling to room temperature. The conversion of styrene and the selectivity and yield of products were calculated by GC-MS.
[0037]
Embodiment 15--24
[0039] (2) The reaction results of different palladium compounds and phosphine ligands for the carbonylation and carboxylation of phenylacetylene to prepare phenylacrylic acid
[0040] Specific experimental steps: Add 0.01mmol of catalyst precursor, 0.012mmol of ligand, 0.05mmol of p-toluenesulfonic acid, 20mmol of phenylacetylene, 5mL of water and nitrogen-methylpyrrolidone (NMP40mL) into 200mL of polytetrafluoroethylene lining in sequence . Put the liner in the autoclave, seal it and check the airtightness of the device, and replace the air in the autoclave with carbon monoxide. Then, carbon monoxide gas was introduced and the pressure was increased to 3.0 MPa, and the reaction was carried out in a constant temperature heating mantle at 120°C for 4 hours, and the pressure was released slowly after cooling to room temperature. The conversion rate of phenylacetylene and the selectivity and yield of products were calculated by GC-MS.
[0041]
Embodiment 25--34
[0043] (3) The reaction results of different catalytic precursors and different ligands for the carbonylation and esterification of styrene to prepare methyl phenylpropionate
[0044] Specific experimental steps: 0.01 mmol of catalyst precursor, 0.012 mmol of ligand, 0.05 mmol of p-toluenesulfonic acid, 20 mmol of styrene, 20 mL of methanol and 40 mL of THF were sequentially added to a 200 mL polytetrafluoroethylene lining. Put the liner in the autoclave, seal it and check the airtightness of the device, and replace the air in the autoclave with carbon monoxide. Then, carbon monoxide gas was introduced and the pressure was increased to 3.0 MPa, and the reaction was carried out in a constant temperature heating mantle at 140°C for 4 hours, and the pressure was released slowly after cooling to room temperature.
[0045] The conversion of styrene and the selectivity and yield of products were calculated by GC-MS.
[0046]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com