Ophthalmic preparation for treating macular edema, optic neuritis and non-infectious entophthalmia through eye drop administration
An ophthalmic preparation and preparation technology, applied in the direction of medical preparations with non-active ingredients, non-central analgesics, medical preparations containing active ingredients, etc., can solve the problems of poor drug delivery, difficulty in effectively treating fundus diseases, and difficult Taking into account safety and effectiveness and other issues, to achieve the effect of small dosage, avoid systemic toxic and side effects, and increase medical compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Embodiment 1, the preparation of ophthalmic preparation of the present invention
[0089] Weigh 0.24g CMC-Na (sodium carboxymethylcellulose, ionic polymer) according to Table 1 and add it to a glass conical flask containing 40mL of pure water, turn on magnetic stirring for 2 hours to obtain solution 1; weigh 1.0g respectively Add polysorbate 80 (surfactant) and 0.24g HPMC (hydroxypropyl methylcellulose, thickener) to a glass conical flask containing 60mL of purified water, turn on magnetic stirring, and heat in a water bath at about 40°C for 1.5 hours. Solution 2 was obtained; 40 mg of dexamethasone and 4 mL of PEG400 (i.e. 4.3 times the amount of surfactant (w / w)) were weighed and put into solution 2, continued heating and stirring for 30 minutes, added solution 1, and stirred for 30 minutes to obtain a mixed solution; Disperse the mixed liquid with a dispersing machine at a speed of 9500 for 5 minutes, stop the machine until the foam disappears, and filter it with a B...
Embodiment 2
[0094] Embodiment 2, the preparation of ophthalmic preparation of the present invention
[0095] The preparation method refers to Example 1, and the raw materials and dosage are shown in Table 1. A colorless and clear solution after removal of impurities was obtained.
[0096] pH adjustment: adjust to pH 6.5 with 0.2N NaOH or / and 0.1N HCl.
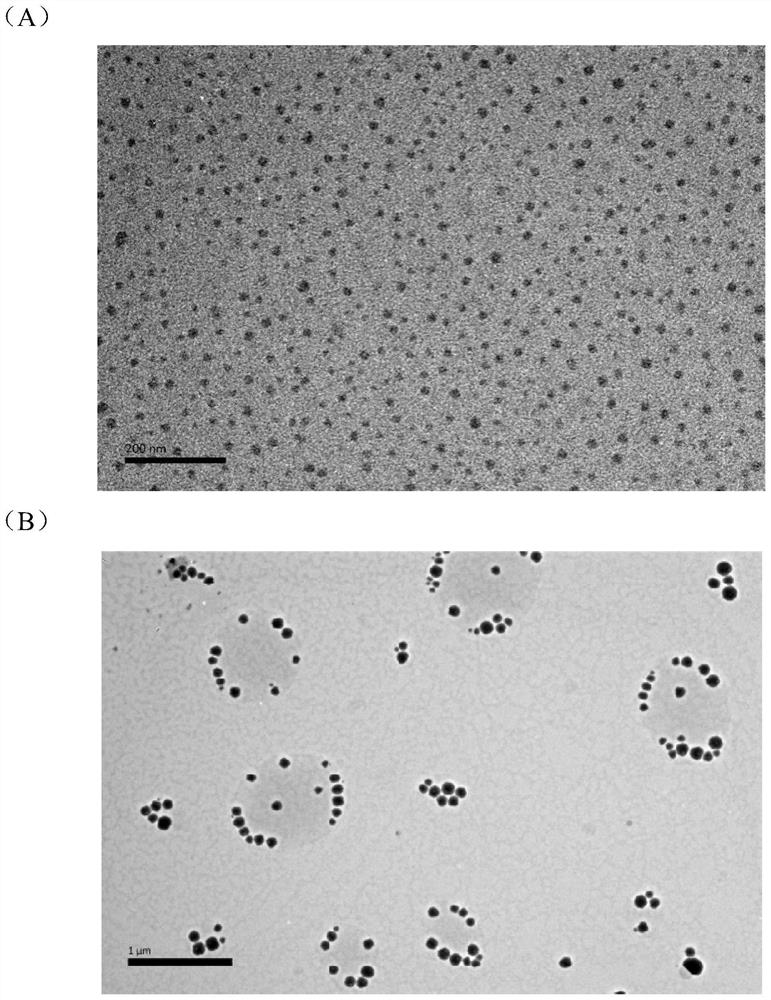
[0097] The HPLC detection method is the same as in Example 1, and the HPLC content detection result: 95.1%, particle diameter 12.9nm (92.1%), PdI: 0.509; stability is good, and the appearance and content have no obvious change after being placed at room temperature in the dark for 1 month; 2 A small amount of precipitation appeared after one month.
[0098] The concentration of API in rat vitreous body 1 hour after eye drops was 13.9ng / g, RSD: 17.2%.
Embodiment 3
[0099] Embodiment 3, the preparation of ophthalmic preparation of the present invention
[0100] Refer to Example 1 for the preparation method and pH and osmotic pressure adjustment method. The raw materials and dosage are shown in Table 1, and a light yellow clear solution after impurity removal is obtained.
[0101] HPLC detection: Column: ZORBAX Eclipse Plus C18, 4.6x100mm 3.5μm; mobile phase A: 0.1% phosphoric acid, mobile phase B: methanol (80:20) isocratic elution, Temp.: 35°C, detection wavelength: 280nm, Flowrate : 0.8ml / min; test result: 98.4%. Particle size: 39.7nm (95.5%), PdI: 0.318; stored in the dark at room temperature for 1 month, no change in appearance and content.
[0102] The concentration of API in rat vitreous was 78.3ng / g 0.5 hours after eye drops.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com