Injectable temperature-sensitive hydrogel capable of treating osteoarthritis
An osteoarthritis and hydrogel technology, which can be used in anti-inflammatory agents, bone diseases, allergic diseases, etc., can solve the problems of infection, unsatisfactory curative effect, high cost, etc., to promote repair, good biocompatibility and The effect of degradability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1. Preparation of hydrogel
[0025] 1. Preparation of stock solutions
[0026] 1) Type I collagen stock solution: use HCl with pH=2 to prepare 5 mg / ml type I collagen;
[0027] 2) Sodium alginate stock solution: use ultrapure water to prepare 2.5% w / v sodium alginate;
[0028] 3) Hyaluronic acid stock solution: use ultrapure water to prepare a 10 mg / ml hyaluronic acid solution;
[0029] 4) Calcium pyrophosphate stock solution: use HCl with pH=2 to prepare 2 mg / ml calcium pyrophosphate solution;
[0030] 5) Sodium bicarbonate stock solution: Use ultrapure water to prepare a supersaturated sodium bicarbonate stock solution with a final concentration of 7.4 g / L.
[0031] 2. Preparation of hydrogel (hydrogel)
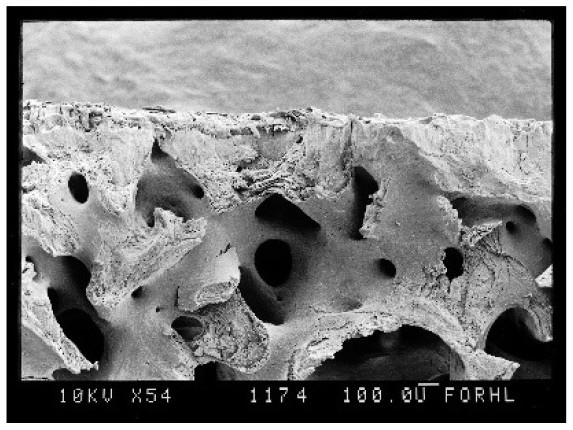
[0032] a) For every 5 ml of type I collagen stock solution, add 1 ul of calcium pyrophosphate and 10 mg of hydroxyapatite particles;
[0033] b) According to 60 ul NaHCO 3 , 100 ul sodium alginate, 240 ul hyaluronic acid stock solution, and 600 ul colla...
Embodiment 2
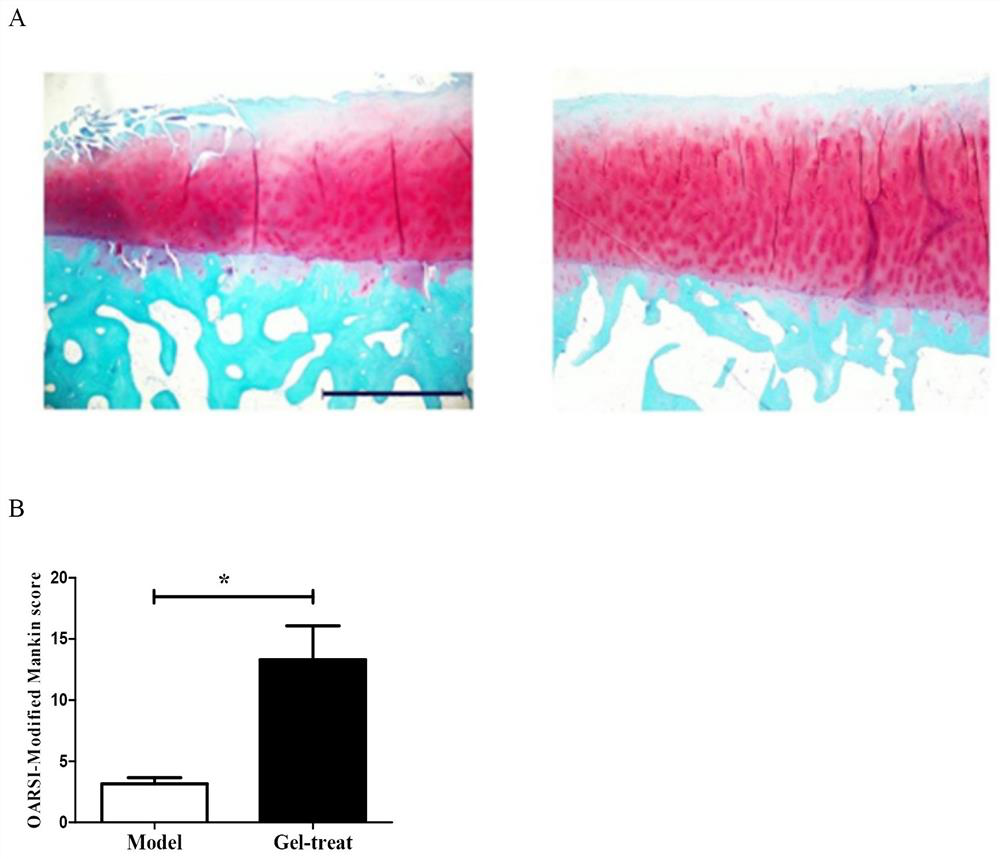
[0036] Example 2 Effect of the gel of the present invention on osteoarthritis
[0037] The SD rats were divided into two groups: model group (model) and treatment group (gel-treat). Animals underwent amputation of the anterior cruciate ligament to establish an osteoarthritis model. That is, SD rats were anesthetized and fixed on a platform, and the leg on the side to be operated was bent at 90°. Cut the superficial skin of the knee with a scalpel, and bluntly dissect the subcutaneous fascia, avoiding subcutaneous blood vessels. Cut the vastus medialis muscle from the junction where the vastus medialis muscle forms the patellar ligament, taking care to avoid intramuscular blood vessels in the vastus medialis muscle to avoid bleeding. The patella is then pushed laterally, exposing the knee joint space. The fat pad under the patella was pulled apart with micro-tweezers, and the anterior cruciate ligament was cut. After the wound was sutured, iodine tincture was applied extern...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com