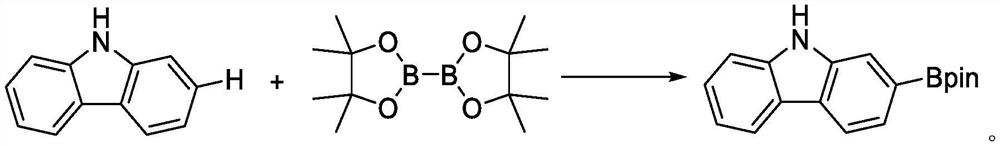
Synthesis method of 9H-carbazole-2-boronic acid pinacol ester by direct C-H boronation of carbazole C2 site
A synthesis method, pinacol ester technology, applied in the field of organic synthesis of carbazole derivatives, can solve the problems of high difficulty in later stage purification, complicated production steps, low utilization rate of atoms, etc., and achieve easy industrial production and low production cost , The effect of high atom utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Synthesis Method of 9H-Carbazole-2-Borate Synthesis
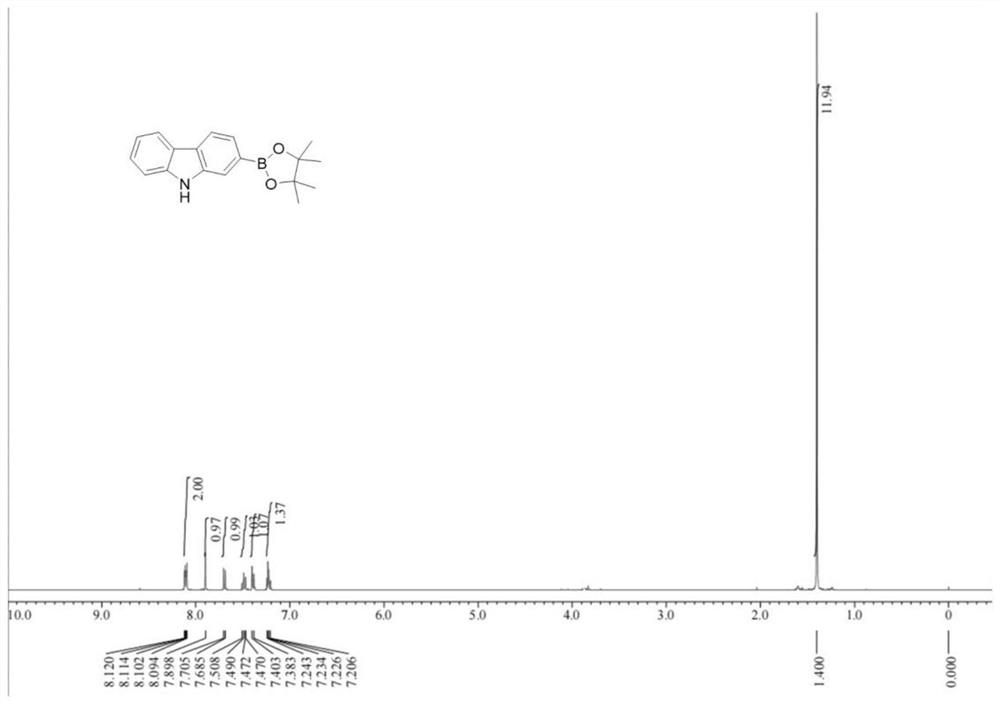
[0037] The synthetic step includes: 50.7 g of carbazole (99%, 0.3 mol), 86.1 g of trifluoroacetate (99%, 0.6 mol), 2.0g [IR (OME) [IR (OME) under nitrogen protection (COD)] 2 (99%, 3 mmol), 0.6 g of ligand L1 (99%, 3 mmol), 23.0 g of alkali Base1 (99%, 0.15 mol) and 300 ml of tetrahydrofuran; the feed is completed, temperature rise to 50 ° C, stirring speed 350 rpm, insulation reaction for 12 hours After the CH boron reaction; after the reaction, water quenching reaction is added, the cooling room temperature filter, the liquid-reduced pressure distillation recovered solvent, petroleum ether crystals, to obtain 75.6 g of 9H-carbazole-2-boric acid-band The content was 99.5%, and the yield was 86.0%. The nervous resonance spectrum of 9H-carbazole-2-boronic acid frequency prepared by the present embodiment is like figure 1 Distance 1 H NMR (400MHz, CDCL 3 : δ = 1.40 (S, 12H), 7.23 (t, j = 7.8 Hz, 1H), 7.39 (D, J ...
Embodiment 2
[0038] Example 2: Synthesis of 9H-carbazole-2-boronic acid-frequency synthesis
[0039] The synthetic step includes: 50.7 g of carbazole (99%, 0.3 mol), 172.2 g of trifluoroacetate (99%, 1.2 mol), 2.0 g of [IR (OME) (COD)] 2 (99%, 3mmol), 1.04 g of ligand L2 (99%, 3 mmol), 23.0 g of alkali base1 (99%, 0.15 mol) and 300 ml of tetrahydrofuran; the feeding is 250 rpm, stirring speed 350 rpm, insulation reaction for 24 hours After the CH boron reaction; after the reaction, water quenching reaction is added, the cooling room temperature is filtered, the liquid is evaporated and evaporated. The content was 99.3%, and the yield was 88.9%.
Embodiment 3
[0040] Example 3: Synthesis of 9H-carbazole-2-boronic acid-frequency synthesis
[0041] The synthetic step comprises: 50.7 g of carbazole (99%, 0.3 mol), 129.2 g of trifluoroacetate (99%, 0.9 mol), 2.0 g of [IR (COD) CL] 2 (99%, 3 mmol), 0.6 g of ligand L1 (99%, 3 mmol), 21.7 g of alkali base2 (99%, 0.15 mol) and 600 ml of cyclopentyl ether; the feed was bonded, heated to 80 ° C, stirring the speed of 350 rpm, The incubation reaction was 24 hours, and the CH boron reaction was performed; after the reaction was completed, water quenched reaction was added, and the cooling room temperature was filtered, the liquid-reduced pressure distillation recovered solvent, the petroleum ether crystal obtained 74.4 g of 9H-carbazole-2-boric acid frequency Which alkoxide, a content of 99.6%, a yield of 84.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com